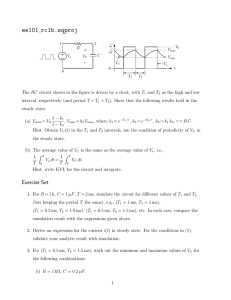
Km 1 Vm [S] 1) v = = v vs. [S] - Hyperbolic 2) Lineweaver Burk
advertisement
![Km 1 Vm [S] 1) v = = v vs. [S] - Hyperbolic 2) Lineweaver Burk](http://s2.studylib.net/store/data/018724715_1-069561ef6a33d2749e373bad86f80405-768x994.png)
Lectures 5 and 6 Inhibition Systems and Derivation of their Rate Equations Vm[S] S +Km = Vmax v 1) v= 2) Lineweaver Burk - 1 v vs v vs. [S] - Hyperbolic 1 [S] Linear Plot or Double Reciprocal Plot Basic Equation V = Vm[S] [S] +Km 1 v = 1 Vmax y = b (TAKE RECIPROCAL) Km + Vm + intercept 1 v 1 [S] m slope Km Vm 1 Vmax ∞, @ [S] 1 Km X 1 [S] 1 [S] O, 1 ∴ Y intercept = Vmax @1 v =O 1 = Vm - Km Vm Cross Multiply 1 1 Km = S 1 S Km 1 Vm x [S] = O v EADIE-HOFSTEE PLOTS - v vs [S] V= 1 v (Gives Km & Vmax directly) Vm[S] [S]+Km Km 1 = Vm + Vm 1 Vmax Multiply by Vmax • v Slope = - Km v Vm = v + Km s v Vmax Km Or rearranging v v = Vm - (Km) x [S] Y= b m x v [S] Rules for deriving rate laws for simple systems 1. Write reactions involved in forming P from S 2. Write the conservation equation for expressing the total enzyme concentration [E]total among the various species 3. Write the velocity dependence equation, summing all the catalytic rates constants multiplied by the concentration of the respective produt - forming species. 4. Divide the velocity dependence equation by the conservation equation. 5. Express the concentration of each enzymic species in terms of free enzyme concentration & substitute 6. Algebra For a simple 1 step reaction, no inhibitor (1) k1[S] E+S k3 ES E+P k2 (2) [E]T = [E] = [ES] (3) v = k3[ES] k3[ES] v [E]T = [E] + [ES] (4) (5) [ES] = k1[S] • [E] - k2[ES] - k3[ES] [ES] = k1[S] • [E] - (k2+ k3)[ES] @ steady state d[ES] dt = O, ∴ rate of formation = rate of breakdown k1 [S] • [E] = (k2 + k3)[ES] [ES] = (6) k1[S]•[E] , k 2 + k3 Define Km = k2 + k3 k1 Substitute into step (4) [S] vv K k33 • Km •[E] [ET] [E ] T = [S] E + Km • [E] = [S]•[E] Km [S] v ÷ Top and bottom = k3 Km = [E ] T By[E] 1+ S Km Multiply by Km v [ET] k3 [S] = ; Km + [S] = [ES] Define Vmax [S] Vmax = k3 [ET] ; v = Km + [S] EQUATION FOR COMPETITIVE INNIBITION • MUTUALLY EXCLUSIVE BINDING OF S AND I k1[S] k3 E +S ES E+P k2 k on [I] k off - Can drive all E to ES By increasing [S] - Since [I] & [S] are mutually exclusive binders, [I] apparently decreases affinity for E, e.g. Km EI [E]T = [E] + [ES] = [EI] v = k3 [ES] v [E]T = k3[ES] [E]+[ES]+[EI] [ES] = k1 • [S] • [E] (k2 + k3) = [EI] = 1 Km [S] = Km • [E] k on [E] • [I] = k off [EI] @ steady-state d[EI] dt dissociation = O; [I] • [E] KI [S] v = k3 Km • [E] [E]T [S] E + Km • [E] + [I] • [E] k on k off 1 KI= i.e., receprocal constant [EI] k3 [S] Km + [S] + Km [I] KI Define Vmax & Vmax [S] Double 1 Collect Terms Reciprocal = [I] v = Km 1+ KI + [S] ; Equation [I] 1 Km 1 Vmax + Vm [S] 1+ KI b+ x m DETERMINING KI FROM SLOPE REPLOT -Measure 1 v vs 1 S @ several [I] increasing [I] X intercept @ 1 = O v ∴ 1 Vmax = Km Vm 1+ I KI I S 1 v [I] = O As [I] , Slope increases 1 v Cross multiply 1 - Km I 1 - Km 1+ K I Slope of 1 vs 1 = Km (1+ [I] s v Vm KI = 1 Km 1+ [I] Km 1 [S] 1 [S] PLOT SLOPE vs [I] SLOPE Km Vm Km Vmax [I] Km Vmax KI Km Km[I] SLOPE = Vmax + Vmax•K I y = b + m x @ SLOPE O (i.e. x-intercept) -Ki Km -Km = • I Vmax Vmax KI Cross Multiply -KI = [I] @ slope = O NON COMPETITIVE INHIBITION k1[S] E+S k3 ES E+P k2 k on I k off k1[S] EI + S ESI k2 [E]T = [E] + [ES] + [EI] + [ESI] v = k3 [ES] v [E]T = k3[ES] [E] + [ES] + [EI] + [ESI] [S] [ES] = Km I • [E] ; [EI] = KI • [E] [ESI] = Cannot easily calculate [ESI] by steady state hypothesis ∴ Most assume equilibrium thus is valid since EI + ESI are in equilibrium (i.e. k3 = O for ESI ∴ k2 > > > k3) THUS: k on [ES] • I k off [ESI] = v [E]T v [E]T ; S • [I] Km = [E] KI S Km •[E] S E+ • E + [I] • E + [I] Km KI KI k3 [S] Km + [S] + Km [I] + [S] KI k3 = • Vmax[S] v = 1+ [I] S + KI 1+ I Km KI [S] • E Km 1 1 = Vmapp Vm I KI 1+ 1 v Slope = Km Vm 1+ [I] KI Increasing [I] [I] =O 1+ 1 =Yint Vmax = 1 Vmaxapp I KI 1 Vmax // -Km I 1 1 @Xint, ∴ 1+ Vmax K S I V=O 1 S Cross Multiply 1 1 = [S] Km Replots to determine KI Slope of 1 1 v, S Slope = y Slope Slope = Km Vm••KI Km Vmax [I] I 1 =Vmax 1+ KI Km Plot = Vm 1+ [I] KI Km + KM[I] Vm VmKI b + m x x int. of slope vs [I] @ Slope = O ∴- Km[I] Km = VmK [I] I Vm Cross Multiply -KI -KI = [I] @ Slope =O yCan also do intercept replot as well 1 1 [I] Y int = Vmaxapp = Vmax + Vmax • K I x m Uncompetitive Inhibition E+S k1[S] k2 Like non-competitive, [S] @ ∞ cannot drive enzyme to ES form k3 ES E+P There is an obligate order of binding First S Then I ESI ∴I should decrease Km by driving reaction E+S ES towards ES formation V = k3 [ES] [E]t = [E]+[ES]+[ESI] [ES] = k1[S] • [E] k2+k3 = 1 Km ESI= Kon[I] • [ES] Koff = 1 KI V = [ET] V = [ET] V = = [S] • [E] Km I • [ES] [S] • [I] KI Km • KI = k3 [ES] [E]+[ES]+[ESI] S k3 Km • [E] E+ S [E] + [S] • [I] Km Km••kI Vm [S] Km+S+ [S][I] Km+KI Factor Taking Reciprocal 1 V = I binds only to ES Km 1 1 Vm[S]+ Vm 1+[I] kI Vm [S] Km+S 1+ [I] KI ∴Slope unaffected Yint decreased by [I] Increasing [I] 1 v [I]=O 1 X int =Y = O; 1 V max 1 1 - Km [S] 1 1+[I]/KI - Kmapp = Km 1 Y int = Vmax 1 To obtain KI, Replot of V vs [I] [I] 1 1 @ [S] = ∞ 1 = Vmax = Y intercept = Vmax 1+ kI v app [I] - X 1 int = Vmax + Vmax••kI - m b 1 Vmaxapp 1 @ X int = - Vmax app = O 1 KIVm 1 Vmax [I] - KI ∴ 1 [I] [] Vm = - Vmax∞ ∞KI - KI = [I] (I) 1+ KI [I] Km 1 1 Vm[S] = Vm 1+ KI I ∴1 • 1 S V = O - 1+ KI Km = COMPLEX INHIBITION E+S k1 k2 ES k3 E+P + + I I αKs KI αKs EI + S βk3 ESI EI + P α = O or ∞ - Competitive α = 1, β = O - Non-Competitive α = 1, O < β < 1 - Partial Non-Competitive 1 < α < ∞, β = 1 - Partial Competitive 1 < α < ∞, β = O - Mixed Inhibition (Type 1) 1 < α < ∞, O < β <1 - Mixed Inhibition (Type 2) In partial inhibition, the EI or ESI complexes are not dead-end complexes as they are in simply inhibition schemes Partial Non Competitive Inhibition k1 E+S + I ES k3 E+P k2 KI KI k1 EI + S ESI βk3 E+P k2 All forms of E (E + EI) combine equally well with S, ∴ Km does not change. Vmax is decreased because a portion of ES is ESI and ESI EI + P is slower V = k3 [ES] + βk3 [ESI] [E]t = [E] + [ES] + [EI] + [ESI] [S] [ES] = [E] Km [EI] = [I] [E] KI [I] [S] I [ESI] = K [ES] = KI Km [E] I [S] [I] V = k3 km E + βk3 K I [F]t E + (÷ ÷) by E (x) by Km Define Vm = k3 [E]t V= [S] E Km [S] [I] [S] + [I] + Km KI KI Km β[I] Vm 1 + KI) [S] _______________________ [I] [I] Km 1 + KI + [S] 1 + KI) V= Vm [S] [I] Km 1 + KI + [S] 1 + βI 1 + KI 1+ [I] KI βI KI Take Reciprocal 1 V 1 Vmax; = [I] Km 1 + KI βI Vm 1 + KI [I] 1+ KI 1 + βI Vmax 1+ KI 1 [S] 1 v [I] = l+ KI Increasing [I] βI Ymax 1+KI [I]=0 @ [I] = ∞ Ks Slope = βVm 1 Y int = βVm 1 Vmax 1 [S] [I] 1+ KI Y intercept = βI Vmax 1+ KI Simplifies to 1 1 = Y int = Ym app Vmax • [I] + KI β[I] + KI Hyperbolic 1 Limit = βVmax Y int = 1 Vmaxapp To obtain βVmax 1 Plot Vmaxapp vs [I] [I] Vmapp = Vmax (β β[I]+KI) [I] + KI Vmapp = βVmax + VmKI [I] multiply both terms B I+Kt I Vmapp = βVm[I] ± VmKI [I]+KI [I]+KI Partial Competitive k1 k2 E+S + k3 ES k2k3 Km= k1 E+P + I I δKI KI αKm EI + S ESI k3 EI V = k3 [ES] + k3 [ESI] [E]t E + EI + ES + ESI [ES] = [S] • [E] Km [I] [I] ESI = αKI • [ES] = αKI •E [EI] = [I] KI The presence of I inhibits on rate of S, ∴ Km is increased (affinity lowered) by a factor α (kα α < 0). Since ESI, EI and E are in equilibrium, and since it makes intuitive sense that S would interfere with I binding, KI is increased by a factor 1 < α < 00. [S] • Km S [I] [S] [E] V = k3 Km • [E] + k3 αKI • Km ÷ by [E] x Km assume Vm=k3 [E]t [S] [I] [I] [S] [E] [E]t [E] + Km + KI • [E] + αKI Km [I] [S] Vm [S] + αKI Km [I] Km + [S] + KI + Factor [I] [S] αKI • Km [I] Vm 1 + αKI S [I] Km 1 + KI [I] + S 1 + αKI Vmax [S] I ÷ by 1 + αKI [I] = 1 + KI Km 1 + [I] + [S] αKI 0r Vmax[S] Km App + [S] Take Reciprocal [I] 1 + KI [I] 1 + αKI Km = Vmax 1 V Slope = [I] Km 1+ KI [I] Vm 1+ αKI 1 [S] Increasing [I] 1 V 1 Vmax Slope = I=0 1 S -1 Kmapp 1 - Km Km Vmax + 1 Vmax [I] 1 + KI [I] 1 + αKI = Km + Vmax I + KI I + αKI = I Km KI I Vm αKI Simplified to: Slope = αKm Vm Hyperbolic Plot limit = αk3 Vmax Slope Makes sense in that in presence of infinite [I] can still bind S, albeit weaker than in absence of I [I]