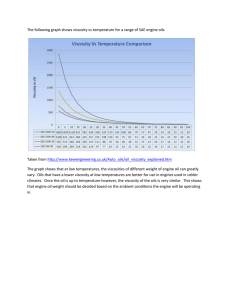
Assessing the antimicrobial activity of essential oils with
advertisement

Assessing the antimicrobial activity of essential oils with MIC and Checkerboard assays Maria Lindewall Bachelor of Health care, Vaasa Program: Biomedical Laboratory Scientist Vaasa / 2015 BACHELOR’S THESIS Author: Maria Lindewall Education and place: Biomedical Laboratory Scientist, Vaasa Supervisor: Ulla Penttinen Title: Assessing the antimicrobial activity of essential oils with MIC and Checkerboard assays _____________________________________________________________________ Date 17.11.2015 Number of pages 50 Appendices 3 ____________________________________________________________________ Abstract Microbes develop more and more resistance to the antimicrobial medications currently on the market. The pharmaceutical development of new medication can’t keep up with the pressure from resistant strains. The solution might be found in nature; essential oils. Essential oils and their components have various antimicrobial activities. They have been used for years for different purposes, but might also be suitable as medication. The use of essential oils could bring natural medication, with fewer side effects and lower toxicity than the drugs available now. The aim with this thesis was to investigate the antimicrobial effects of essential oils. Minimal Inhibitory Concentration is one of the methods chosen and was used to assess the antimicrobial activities. Checkerboard assay was used to examine the interactions between different essential oils or essential oil components. Candida albicans, Candida glabrata, Staphylococcus Aureus and Pseudomonas aeruginosa were investigated with the two assays. Antimicrobial effects are shown in every fungal assay. Antifungal activities were found when investigating C. albicans and C. glabrata. The results on S. aureus and P. aeruginosa were not conclusive, and need more research. ____________________________________________________________________ Language: English Key words: Essential oils MIC Checkerboard _____________________________________________________________________ EXAMENSARBETE Författare: Maria Lindewall Utbildning och ort: Bioanalytik, Vasa Handledare: Ulla Penttinen Titel: Bedömning av den antimikrobiella aktiviteten hos essentiella oljor med metoderna MIC och Checkerboard _____________________________________________________________________ Datum 17.11.2015 Sidantal 50 Bilagor 3 _____________________________________________________________________ Abstrakt Mikrober utvecklar mer och mer resistens mot de antimikrobiella läkemedel som finns på marknaden. Den moderna läkemedelsutveckligen kan snart inte motverka detta. Lösningen finns kanske att finna i naturen; essentiella oljor. Essentiella oljor och deras komponenter har ett flertal antimikrobiella aktiviteter. De har blivit använda länge för olika ändamål, men kan också komma att bli viktiga för framtida läkemedel. Användningen av essentiella oljor kan ge naturliga läkemedel, med mindre bieffekter och lägre toxicitet än dagens mediciner. Målet med detta examensarbete var att undersöka de antimikrobiella effekterna hos essentiella oljor. Minimal Inhibtory Concentration har använts som analysmetod och interaktioner mellan olika essentiella oljor och essentiella oljors komponenter har undersökts med Checkerboard analys. Candida albicans, Candida glabrata, Staphylococcus Aureus och Pseudomonas aeruginosa blev undersökta med dessa två analyser. I varje experiment där jästsvamparna undersökts, fanns det att finna antimikrobiella effekter av de essentiella oljorna. Antimykotiska effekter fanns hos både C. albicans och C. glabrata. Resultaten med S. aureus och P. aeuruginosa blev inte avgörande, och kräver mera forskning. _____________________________________________________________________ Språk: Engelska Nyckelord: Essentiella oljor MIC Checkerboard _____________________________________________________________________ OPINNÄYTETYÖ Tekijä: Maria Lindewall Koulutusohjelma ja paikkakunta: Bioanalyytikko, Vaasa Ohjaaja: Ulla Penttinen Nimike: Eteeristen öljyjen antimikrobiaktiivisuuden määrittäminen MIC ja Checkerboard menetelmillä Päivämäärä 17.11.2015 Sivumäärä 50 Liitteet 3 _________________________________________________________________________ Tiivistelmä Mikrobilääkeresistenssi on viime vuosina lisääntynyt huomattavasti. Nykypäivän lääkekehitys ei pysy samassa tahdissa resistenssin kanssa. Ratkaisun tähän saattavat tuoda luonnosta löytyvät eteeriset öljyt. Eteerisillä öljyillä ja niiden aineosilla on antimikrobisia vaikutuksia. Eteeriset öljyt ovat olleet käytössä eri tarkoituksissa pitkään. Tulevaisuudessa nämä öljyt voisivat toimia lääkkeinä. Eteeriset öljyt saattaisivat olla luonnollisia lääkeaineita joilla olisi vähemmän sivuvaikutuksia ja alempi toksisuus kuin nykypäivän lääkkeillä. Opinnäytetyössä on tutkittu eteeristen öljyjen antimikrobista vaikutusta Minimal Inhibtory Concentration (MIC) –menetelmällä. Eteeristen öljyjen ja aineosien yhteisvaikutusta on tutkittu Checkerboard (CB) -menetelmällä. Tutkimuksessa käytettiin seuraavia mikrobeja Candida albicans, Candida glabrata, Staphylococcus Aureus ja Pseudomonas aeruginosa. Eteeristen öljyjen vaikutus oli nähtävissä kaikissa kokeissa joissa käytettiin sienipatogeenejä. Sekä C. albicans että C. glabrata -kokeissa nähtiin tuloksena antimykoottisia vaikutuksia. S. aureus ja P. aeruginosa -kokeiden tulos jäi osittain epäselväksi ja vaati lisää tutkimuksia. Kieli: Englanti Avainsanat: Eteeriset öljyt MIC Checkerboard _________________________________________________________________________ Table of Contents 1 Introduction ................................................................................................................................................ 1 2 Aims of the Study .................................................................................................................................... 2 3 Theoretical Background ......................................................................................................................... 3 3.1 Microbes ............................................................................................................................................. 3 3.1.1 Candida albicans (C. albicans) ........................................................................................... 3 3.1.2 Candida glabrata (C. glabrata) .......................................................................................... 4 3.1.3 Staphylococcus aureus (S. aureus) ..................................................................................... 5 3.1.4 Pseudomonas aeruginosa (P. aeruginosa) ...................................................................... 5 3.2 Antimicrobial resistance and biofilm formation ..................................................................... 6 3.3 Essential oils (EOs) ......................................................................................................................... 8 3.3.1 Bioactivity of essential oils................................................................................................... 9 3.3.2 Essential oil components .................................................................................................... 11 3.3.3 Interactions between essential oil components ............................................................ 13 3.4 Investigating the antimicrobial effect of essential oils....................................................... 14 3.4.1 The Agar Diffusion Method .............................................................................................. 15 3.4.2 The Dilution Method ........................................................................................................... 15 3.4.3 Checkerboard Method (CB) .............................................................................................. 17 3.4.4 Other methods ....................................................................................................................... 18 3.4.5 Growing microbes with the right medium .................................................................... 19 4 Materials and Methods ........................................................................................................................ 21 4.1 Minimal Inhibitory Concentration (MIC) .............................................................................. 25 4.2 Checkerboard Assay (CB) .......................................................................................................... 27 5 Results ...................................................................................................................................................... 31 5.1 Minimal Inhibitory Concentration ........................................................................................... 31 5.2 Checkerboard Assay .................................................................................................................... 36 5.2.1 Candida albicans with C2 and C1B or E79 (CB assay) ........................................... 36 5.2.2. C. albicans and C. glabrata with C2 and E79 (CB assay) ...................................... 37 5.2.3 C. glabrata with C2 and E79 (CB, kinetic assay) ....................................................... 42 5.2.4 S. aureus and P. aeruginosa with C2 and E79 (CB assay) ...................................... 44 6 Discussion and Conclusion ................................................................................................................ 45 7 References ............................................................................................................................................... 47 Appendices .................................................................................................................................................... 1 1 1 Introduction During the development of pharmaceuticals, humans have defeated a lot of pathogens thanks to medications. Despite the huge success with these antimicrobial medications, microbes have been and are developing resistance against our treatments. It’s a tight race between new drugs and new resistant strains. Due to the strong antimicrobial medications, more aggressive microbes are evolving than earlier. (World Health Organization, 2015). Infections that could have been fought off easily, are now being replaced by infections caused by resistant strains. Bacterial diseases that where treated with a simple antibiotic, might now require a much longer treatment with stronger antimicrobial drugs with more side effects than before. (WHO, 2015). Resistant strains need to be treated with new medication that has lower toxicity and fewer side effects than today’s drugs. Developing newer and stronger antimicrobial drugs usually only triggers more resistance. It is safe to say that the world is moving onto a postantibiotic era. (Kalemba & Kunicka, 2003, 813). A new and exciting idea has been forming over the past few years. Essential oils, produced by various aromatic plants, have been known for their antimicrobial effect for a long time. They have been utilized as food preservatives and aromatherapy as well as in make-up and perfumes. An increase in interest in these plants’ possible antimicrobial activity has sparked research about essential oils. (Kalemba & Kunicka, 2003, 813). Essential oils, or their components, could be the new line of medication against various pathogens. Essential oils are natural and complex compounds, with several activities that can be used in fighting infections. (Kalemba & Kunicka, 2003, 813). This Bachelor’s thesis will give a better view on essential oils and their possible antimicrobial activity. 2 2 Aims of the Study There are a lot of studies made on essential oils and their activities. The goal with this thesis is to present facts about essential oils, discuss different ways to examine them and assess the activity through chosen assays. Altogether, four different assays were used during the period of investigation. All of these assays were made in order to get a better idea of what kind of effect the essential oils have. One of them was a novel assay that has not been reported in any literature. Due to this, the nature of this assay will not be discussed here. The Minimal Inhibitory Concentration (MIC) assay is one of the assays described and used. The MIC assay is a typical assay for antimicrobial drugs and it seems natural to use it with essential oils as well. This assay was made to check how active the antimicrobial agent is at killing off microbes, and at what concentrations. The goal was to get a good overview on how essential oils or their components killed off chosen microbes. The second assay important to this thesis was the Checkerboard (CB) assay. Two different antimicrobial agents are examined at once, and the interaction between the two is of interest. The goal was to find a good interaction with one component and optimise the assay for both fungi and bacteria. A biofilm assay was also made in order to see the effect of essential oils and their components on a biofilm. Unfortunately, there was not enough time to optimise the assay and therefore the results are not presented. The main goal of this thesis is to show the antimicrobial effect of essential oils or their components with different kinds of assays on four known pathogens. The microbes chosen were Candida albicans, Candida glabrata, Staphylococcus aureus and Pseudomonas aeruginosa. All of these pathogens have a clear infection pattern in humans, causing a lot of infections and are also known for developing resistance. The main questions for this thesis have been: “What are essential oils?” “How can the antimicrobial effect of essential oils be analysed?” “Do essential oils and their components have antimicrobial activity”? 3 3 Theoretical Background The main theoretical points behind this Bachelor’s thesis will be discussed in this chapter. The most important information is the knowledge about essential oils. Other important knowledge is about the microbes investigated, resistance to antimicrobial medication, biofilm formation, difficulties with examining essential oils and different analyses to assess the antimicrobial function of essential oils. 3.1 Microbes There are two main microbes of interest in this Bachelor’s thesis; Candida albicans and Candida glabrata. The two other microbes examined are Staphylococcus aureus and Pseudomonas aeruginosa. These pathogens cause common infections in humans. In the following chapters basic information about these pathogens will be presented. 3.1.1 Candida albicans (C. albicans) Candida albicans is one of the 600 species of fungi that are considered pathogens to human beings. Of all the Candida species, C. albicans is the most studied one. C. albicans is a eukaryotic cell, making it very similar to human cells. It is a diploid fungus and can switch from yeast to hyphal growth. C. albicans is an opportunistic fungus that can live in the genitourinary tract, mouth and skin. It is harmless but can cause an infection when the host is experiencing a lowered immune system, a disease or an imbalance of the microbiota. The infections C. albicans can cause vary from superficial infections to systematic and life-threatening infections. Usually a fungal infection of the mouth, oral candidiasis, is the first sign that a person suffers from human immunodeficiency virus (HIV). Other infections that C. albicans cause are e.g. vaginitis, an inflammation of the vagina. This affects around 75% of women at least once during their lifetime, with 40-50% having a second or more episodes of the infection. In more serious cases, C. albicans can infect the blood stream. C. albicans is now causing about a third to a fourth of all nosocomial infections. (Berman, 2012, p. R620; Granger, 2012, 795; Mayer & Wilson & Hube, 2013, 119). 4 Antimicrobial medication, medical implants and immunosuppressive treatment makes C. albicans infections more common. A broad-spectrum antibiotic used for a bacterial infection, can allow the fungus to take over. Trauma or gastrointestinal surgery will also increase the risk of a serious fungal infection. (Huovinen & Meri & Peltola & Vaara & Vaheri & Valtonen, 2003, 299; Mayer et.al., 2013, 119). C. albicans has numerous virulence factors important to its ability to infect human beings. The virulence factors are e.g. adhesins on the cell surface, biofilm formation, ability to switch between different morphological states, pH sensing and regulation as well as metabolic adaption. (Granger, 2012, 795; Mayer et.al., 2013, 120-125). There are only three major groups of antifungal drugs on the market. First one are polyenes, such as nystatin and amphotericin B. The second antifungal group is azoles, e.g. fluconazole. The third group are enchinocandins, where micafungin and caspofungin can be mentioned. (LaFleur, 2011, 22-23). The antifungal drugs on the market today usually have a high toxicity to the host, cause resistant strains and are expensive. The side effects include nephrotoxicity, hepatoxicity and gastrointestinal distress. New drugs need to be low-cost, eco-friendly and broadspectrum. These reasons make it important to look for other, more natural medications. (Rajeshkumas & Sundararaman, 2012, e60-e61). 3.1.2 Candida glabrata (C. glabrata) Candida glabrata is a haploid fungus that can be found in human microbiota. C. glabrata acts opportunistically. It can be found in the same areas as C albicans and causes infections during similar conditions. (Brunke & Hube, 2013, 701). C. glabrata is becoming a common fungal pathogen due to the ever increasing use of antimicrobial agents and immunosuppressive treatments. The infections caused by C. glabrata are difficult to treat and usually resistant to antifungal medication, especially fluconazole. This Candida species is not as studied as C. albicans at the moment, even though it is growing as one of the leading causes of fungal infections in human beings. (Fidel & Vazquez & Sobel, 1999, e80) 5 3.1.3 Staphylococcus aureus (S. aureus) S. aureus is a gram-positive coccal bacterium that causes a lot of different infections in humans. The bacteria can live in our microbiota, mainly in the nose and throat area as well as on the skin. If the bacterium finds its way into an open wound it can cause a skin infection. The bacterium is spread via direct or droplet contact. S. aureus can infect surgical wounds and cause infections throughout the body. It can also cause sepsis and endocarditis. (Huovinen et.al., 2003, 98-102). Foster (2004, 1693-1696) claims that S. aureus is a “superbug”, due to its virulence factors and ability to fight antibiotic agents. The virulence factors that S. aureus have include surface proteins promoting adherence to tissue. These surface proteins can bind proteins in the blood to escape the immune response and promote iron intake. Another virulence factor is membrane-damaging toxins that will cause cell damage and give the symptoms of septic shock. Over 80 % of the strains are resistant against penicillin. MRSA is short for Methicillinresistant Staphylococcus aureus and is a multi-resistant strain of S. aureus. This strain carries a mecA gene that contains the resistance. MRSA is very common in nosocomial infections, and spreads easily from patient to patient. The only antibiotics available to treat MRSA are vancomycin and teicoplanin. Even with treatment, the bacterium is hard to eliminate altogether and one might carry the strain in the microbiota for years. (Huovinen et.al., 2003, 98-103; Gibbons, 2003, 263). 3.1.4 Pseudomonas aeruginosa (P. aeruginosa) P. aeruginosa is a bacillus shaped bacterium, with one flagellum. P. aeruginosa can grow both in aerobic and anaerobic conditions. You can find it in the soil, water, plants, humans and animals and it has a high adaptability to life. When infecting a human, common colonisation areas are perineum, underarms and ears. (Huovinen et.al., 2003, 193). P. aeruginosa is an opportunistic bacterium. It rarely infects healthy human beings. One can get infected from staying at a hospital, especially if there is an open wound of some kind. P. aeruginosa has a lot of virulence factors that it uses to its benefit. The bacterium can attach to different parts of the host’s cells and form biofilms. Common infections caused by P. aeruginosa are ear infections, urinary tract infections, skin infections, chronic 6 pneumonia for patients with cystic fibrosis, keratitis, infections in the gastrointestinal tract, endocarditis, meningitis and bacteraemia. (Huovinen et.al., 2003, 196-198). P. aeruginosa is naturally resistant to a lot of antibiotics. This is possible thanks to the outer membrane of the bacterium. P. aeruginosa also has a natural resistance to some of the essential oils due to the cell wall construction. This makes P. aeruginosa an interesting bacterium to examine with essential oils. (Huovinen et.al., 2003, 199; Kalemba & Kunicka, 2003, 818). 3.2 Antimicrobial resistance and biofilm formation Antimicrobial resistance is a common problem in today’s world. Antimicrobial resistance means that a drug that was usually effective to one microbe, no longer has any effect. The microbe has developed a resistance to the used drug. This resistance occurs from errors in replication or an exchange of resistance genes. The misuse of antibiotics also increases the resistance. (World Health Organization, 2015). Antimicrobial resistance is found in bacteria (antibiotic resistance), fungi, viruses and parasites. Resistance is mostly found in pathogens causing common infections. A resistance-type disease will prolong the treatment time. Infections caused by a resistant strain infects others easier than an infection caused by the same non-resistant microbe. The resistance strains found in hospitals are usually highly resistant, like MRSA (MethicillinResistant Staphylococcus aureus) and Extended Spectrum Beta-Lactamases ESBLproducing gram negative bacteria. (WHO, 2015) The antimicrobial resistance is a threat to the global health. The resistance makes it nearly impossible to treat diseases, even fairly common ones. New methods of treating microbes are being evaluated, since it is hard to develop new effective antibiotics. (WHO, 2015). It is interesting to see if essential oils could be a solution to this. Antibiotic-resistant strains are of interest when examining essential oils’ bioactivity. There is also hope that essential oils could defeat biofilms. It is believed that these oils would give fewer side effects than the man-made antimicrobial agents in the market right now. (Raut & Karuppayil, 2014, 252). 7 Biofilm is a formation where single-celled bacteria or fungi come together, adhere to a surface and start a complex community together. Biofilm formation can grow on surfaces like catheters, dental prostheses and other medical devices. The biofilm has a complex structure and is more resistant to antimicrobial medication than planktonic cells (cells that don’t exist in biofilm form). Hence the biofilm is becoming a crucial obstacle in treating patients and is studied continuously. (Bordi & Bentzmann, 2011, p. 1-8; Berman, 2012, R620). The biofilm requires the planktonic cells to change into a biofilm lifestyle. The microbes adhere to a surface and to each other. The biofilm forms due to a variation of pathways, ignited with environmental signals. Functions within the biofilm is controlled by either chemical or genetic communication. (Bordi & Bentzmann. 2011, 1). There can be several species in the biofilm, which makes every biofilm unique. The biofilm gives the microbes a chance to work together instead of competing with each other. Horizontal gene transfer and crossbreeding of genes will happen and add to the variety of the biofilm. Oxygen deprivation and starvation cause stressful environment for the microbes and this will make a cultivation of new subpopulations to arise. (Bordi & Bentzmann. 2011, 1-2). There is a resistance for many antimicrobial drugs and immune responses within the biofilm. The matrix of the biofilm makes antibiotic diffusion harder. A mixture of molecules can also cause a physical barrier to drugs. This makes the biofilm extremely hard to treat. It is also difficult to correctly diagnose a biofilm. There is no good sampling method in use. In successful treatments, the cure has been based on a lack of symptoms of the patient and negative cultures. (Bordi & Bentzmann. 2011, 1-3). Several approaches have been tried out to eliminate biofilms. Iron is important to the life of the microbes and therefore iron deprivation could be utilized. Changing the critical pathways or interfering with the adhesion process could also work. New treatments are being examined, like the use of essential oils as medication. (Bordi & Bentzmann. 2011, 6) 8 3.3 Essential oils (EOs) Essential oils are natural compounds produced by a wide range of aromatic plants. Essential oils have been used for a long time as food preservatives, in perfumes and makeup and as remedies, thanks to their antiseptic and bactericidal effect. (Kalemba & Kunicka, 2003, 813; Bakkali & Averbeck & Averbeck & Idaomar, 2008, 446-447). Essential oils are volatile, liquid, and hydrophobic. The odour of the oils is strong and the colour is usually clear, sometimes vaguely coloured. The oils are secondary metabolites and have a low molecular weight. There are around 3000 known essential oils, of which 300 are important from the commercial point of view. The essential oils consist of different components. (Lahlou, 2004, 435; Bakkali et. al, 2008, 447). Essential oils are produced by aromatic plants in the Mediterranean and tropical countries. The oils are produced by all plant parts e.g. flowers, buds, stem, leaves, seeds and fruits. The essential oil protects the plant from bacteria, viruses and insects. They give a certain taste to the plant and can therefore also protect the plant from herbivores. (Bakkali et. al, 2008, 447). The oils can be derived with various techniques; hydro-distillation, steamdistillation, hydrodiffusion or CO2 extraction. Other techniques that have been reported are microwave irradiation and mechanical and thermochemical reaction. The most used one is hydro-distillation. (Lahlou, 2004, 436). Many of the commercially important essential oils are chemotyped by gas chromatography and mass spectrometry analysis. These techniques are used to identify and compare the components. Also small changes in the chemotype of the same essential oil can now be examined. (Saad & Muller & Lobstein, 2013, 271). It is tricky to get the exact same product when deriving essential oils. The final composition of the oil may differ according to where the plant is harvested, what part of the plant the essential oil is produced by and what kind of derivation method has been in use. It would be important to have a standardized way of choosing the plant organ, when to harvest and so on. This way, the oils could be comparable. (Lahlou, 2004, 436; Bakkali et al., 2008. 447). There are around 60 families of plants producing essential oils. The family Apiaceae is known for its antibacterial, antifungal, antiviral and anticancerous activities. Other important families are Alliaceae, Asteraceae, Myrtacea, Poaceae and Rutaceae. (Raut & Karuppayil, 2014, 250-251). 9 The interest in using essential oils as treatment has grown over the past years. This is due to the ever-growing problem of drug-resistant microbes. The essential oils have natural antibacterial and multiple other bioactivities and could favourably be used as medication. With the population demanding more natural drugs, essential oils might be the answer. They have fewer toxicity side effects, easy to obtain and a better biodegradability than antibiotics and other drugs used. (Kalemba & Kunicka, 2003, 813). 3.3.1 Bioactivity of essential oils Essential oils have been used for a long time as herbal medication and in aromatherapy. They have been used for their antiseptic property against infections of various origins. Essential oils can be used to protect the food from foodborne diseases. The bioactivity of essential oils has been widely examined, but often lack some important information, like the exact origin of the oil. Since one essential oil can differ slightly from another one, depending on harvesting time, plant organ and climate, there will be different results. (Saad et.al., 2013, 271; Flores & Beck & da Silva, 2015, 1). Only a few of the articles read for this thesis explained exactly how the essential oil was extracted. (Toloza & Zygaldo & Biurrun & Rotman & Picollo, 2010, 3; Gutiérrez & Werdin-González & Stefanazzi & Bras & Ferrero, 2015, 1). The bioactivity of essential oils and their components are not yet fully understood. It’s hard to specify a few activities since there is such a wide range of essential oils available. It is proven that essential oils can have antibacterial, antifungal, antiviral, antimutagenic and anti-inflammatory effects as well as being antioxidants. Essential oils are cytotoxic and have no specific cellular target. The effects depend on both what essential oil and microbe are in question. (Kalemba & Kunicka, 2003, 818; Bakkali et. al., 2008, 450). The essential oils have a lipophilic character. This will make them pass through the cell wall and cytoplasmic membrane. The essential oils can then permeabilize other membranes within the cell, destroying most of its structures. Ion loss and reduction of membrane potential will cause the bacteria or cell to death by necrosis or apoptosis. Essential oils can also damage the cell wall, making the cellular content leak. They can coagulate the cytoplasmic membrane, damage lipids and proteins and also inhibit the production of DNA, RNA, proteins and polysaccharides. In eukaryotic cells the target is mostly mitochondrial membranes, which will disrupt the respiratory pathways. What kind of 10 destructive action the essential oils have depends on the EO and microbe, and also concentration and dose. (Kalemba & Kunicka, 2003, 818; Bakkali et. al, 2008, 450). The antibacterial effect is one of the most important and most studied effect. Due to the cell wall construction, the essential oils have different effects when it comes to gram negative and gram positive bacteria. Gram negative bacteria are resistant to a lot more essential oils due to their natural cell wall. Gram positive bacteria are more sensitive. Especially investigating the drug-resistance strains with essential oils is of interest. (Kalemba & Kunicka, 2003, 818-819). The most effective components against bacteria are the phenolic ones, like thymol, carvacrol and eugenol. Garlic, tea-tree, cinnamon and lemon grass oil have been active against MRSA. (Raut & Karuppayil, 2014, 252). Essential oils have shown to have antifungal activity. Fungal infections are becoming a serious health problem. Since the cell type is eukaryotic it is tricky to target these cells without damaging our own cells. A lot of strains have developed resistance and can form biofilms. Finding a new antifungal drug would be a great achievement. For C. albicans, cinnamon, lemongrass, Japanese mint, ginger grass, geranium, and clove oils were shown to inhibit the growth. Terpenoids seem to be the most efficient essential oil component against fungi. The pathways involved in hyphae morphogenesis seem to be stopped by EOs. (Raut & Karuppayil, 2014, 252-255). Antiviral effects have been proven on Epstein - Barr virus and Herpes Simplex Virus. Eucalyptus and thyme oils were effective against HSV. The essential oil interfere with the envelope structures of the virus and thereby disrupt the entry of the virus into the host. (Lahlou, 2004, 442; Raut & Karuppayil, 2014, 257). Anticancerous effects have also been found. Essential oils could be used as preventive medication. It could also shrink the malignancies. The oils interfere with cell signalling, ion channels and membrane functions. (Raut & Karuppayil, 2014, 256-257). Perillyl alcohol (POH) is a monoterpene that has been proven to inhibit cancer cells in cell cultures. This component was put in soft gelatine capsules with soybean oil in order to treat cancer. The pills were taken several times a day. The side effects were intestinal problems, nausea, fatigue and vomiting. Many patients decided to interrupt the trial before any results could be obtained. Later on, another study was performed where POH was taken as a nasal treatment for patients with gliomas. This resulted in decreased tumour size. The nasal 11 treatment had fewer side effects and a rapid absorption. (Chen & Fonseca & Schöntal, 2015, 1580-1585). Ageing, cancer, diabetes and asthma can all be linked to the oxidative stress caused by free radicals. A balance can be obtained by antioxidants. Essential oils show great antioxidant effect. Thymol and carvacrol are strong antioxidants. (Raut & Karuppayil, 2014, 258). Some protozoal infections cause problems with medications. Issues with treating protozoal infections are the long treatment, side effects and drug resistance. Antiprotozoal effects of the essential oils have been found, e.g. oregano oil can cause cell lysis in the trypanosomal parasite. (Raut & Karuppayil, 2014, 2258). Other effects of essential oils that have been reported are anti-inflammatory, anti-diabetic, antimutagenic (Raut & Karuppayil, 2014, 257-259), together with antidiarrhoeal, antinociceptive, insecticidal and gastroprotective activity. It is also said that essential oils could have sedative effects (Lahlou, 2004, 442-444) How do the essential oils effect our body? Since essential oils seem to attack both prokaryotic and eukaryotic cells, there can be some impact on our own cells. Allergies, irritation, toxicity and carcinogenicity are all risks linked to essential oils. Many articles fail to discuss this matter. A complete toxic mapping should be done for essential oils. The problem stays the same here; as some compositions can vary even within the same EO, it’s not always clear that the toxic effects are the same for all the variations. Toxicity cases in humans are linked to ingestion of essential oils and exposure to the skin. (Raut & Karuppayil, 2014, 259) 3.3.2 Essential oil components An essential oil is made up by several components, around 20-60 of them. There are usually two or three major components that make up for a big part of the oil. The rest are minor components. The major components decide what kind of biologically important properties the essential oil will have. The main group of components are terpenes and terpenoids and the other main group is aromatic and aliphatic components. The two enantiomers of an essential oil can have very different bioactivity. (Bakkali et. al, 2008, 447; Saad et.al., 2013, 269; Flores et.al., 2015, 2). 12 The biggest group of essential oil components are the terpenes. They are formed by several isoprenes (5-carbon-based units). They can be divided into other groups, where monoterpenes and sesquiterpenes are the most common ones. Other groups of terpenes are hemiterpenes, diterpenes, triterpenes and tetrapenes. When a terpene contains an oxygen, it is called a terpenoid. (Bakkali et. al, 2004, 449). Monoterpenes consist of two isoprenes. This group of components make up 90% of all the essential oils. There is a great variety of this type of terpenes. Thymol and carvacrol are the most examined components, with great antibacterial effect. Also linalool, citronellol, menthol and 1,8-cineole can be mentioned as antimicrobial. (Bakkali et. al., 2004, 449). The aromatic and aliphatic compounds (like cinnamaldehyde and eugenol) occur less frequently than terpenes and terpenoids. See the most studied components in appendix 1. Two components are shown in picture 1. (Bakkali et. al., 2004, 449). Overall, components containing phenols or aldehydes (e.g. thymol, carvacrol, eugenol and cinnamaldehyde) have the most effective antimicrobial effect. (Bassolé & Juliani, 2012, 3991). The lipophilic and hydrophilic characters play an important part in how antimicrobial the components are. The activity is the highest in phenols and after that the activity goes as follows; aldehydes, ketones, alcohols, ethers and hydrocarbons. (Kalemba & Kunicka, 2003, 824). Picture 1. Thymol and carvacrol, the strongest antimicrobial essential oil components. Find more components and information in appendix 1. Pictures from: https://pubchem.ncbi.nlm.nih.gov/search/search.cgi 13 3.3.3 Interactions between essential oil components All of the components of an essential oil contribute somehow to the overall effect. The major components are usually the ones that decide what kind of bioactivity an essential oil will have, but the minor components also have a significance. Is it the special mixture of components that give the overall effect or only the major components? It might be so that the trace components help or increase the effect of the major ones. The major components, typically the oxygenated ones, show a stronger effect on their own than in an essential oil. In some cases, the oil itself gives a stronger effect. (Kalemba & Kunicka, 2003, 824; Bakkari et. al, 2004, 466; Bassolé & Juliani, 2012, 3989-4006) The interaction between different oil components can be of four different kinds: antagonistic, synergistic, additive or indifferent. Antagonism means that the effect of one or both components are less when combined together than used separately. Synergism gives a better effect when two components are used together than used individually. Additive interaction means that the effect is same when the two components are put together than apart. Indifferent means that there is no interaction at all. (Bassolé & Juliani, 2012, 3991). Essential oils and their components have been examined to see the effect and different interaction tests have been made to determine this. Unfortunately, there is no absolute way to investigate this. Checkerboard assay, Graphical and Time-kill methods are used as methods to test essential oil components’ interaction. All of these methods were originally made for testing interaction between two drugs, but are now also used for the essential oils and their components. So far there is no own standardized way of testing the interaction specifically between two essential oil components. (Bassolé & Juliani, 2012, 3994) Essential oils and components could also have a synergistic effect with antibiotics. The EO could enhance the antibiotic efficacy with improving the diffusion of the antibiotic or to hinder efflux pumps in gram negative bacteria. Thanks to the multicellular targets of EOs, it will most likely be difficult or impossible for the bacteria to develop resistance against EOs. Synergistic effects have been proven, but the underlying mechanism of the synergism has not been fully examined. One problem is also how the EO would be administrated into the human body. (Langeveld & Veldhuizen & Burt, 2014, 76-92). Some ways have already been tested, e.g. capsule form or nasal treatment (Chen et.al., 2015, 1580-1585). 14 Finding synergism between two essential oil components would be beneficial to the market. Less amount and lower concentrations of the essential oils or components could be used to obtain the same result, giving fewer side effects. In the food industries, a synergism between two essential oil components would reduce the flavour that essential oil usually gives. (Kalemba & Kunicka, 2003, 823; Bassolé & Juliani, 2012, 3994). 3.4 Investigating the antimicrobial effect of essential oils Essential oils are hard to examine due to their inability to solute in water. It is critical to make sure that everything is properly mixed together, for the reproducibility of the experiment. An adapted solvent needs to be used to study the biological and pharmacological properties. It has been hard to find a standardized solvent for essential oils. Several solvents have been used in different studies; alcohol, acetone, ethylene glycol, ethanol, methanol, dimethyl sulfoxide (DMSO) and dimethylformamide (DMF). An emulsifier can also be used, e.g. Tween 20 or Tween 80. Whatever solvent is chosen, it will affect and alter the final result. (Lahlou, 2004, 437). Dimethyl sulfoxide is a highly polar but stable product. DMSO is used for research only. It has mild skin and eye irritation features. (ThermoFisher Scientific, Safety Data Sheet). DMSO and ethanol are used in a lot of assays determining MICs and antimicrobial activity. Unfortunately, some interference with assays have been reported when it comes to DMSO. (Wadhwani & Desai & Patel & Lawani & Bahaley & Joshi & Kothari, 2008, 1). There are several ways to measure if an essential oil has an antimicrobial effect. Most of the methods used are taken from assays designed for drug testing. These assays have then been modified a bit to suit the essential oils. A method only dedicated to essential oils has not yet been invented. (Kalemba & Kunicka, 2003, 814). The growth of microbes is usually verified with turbidity. The growth can be measured with an instrument that recognises changes in the optical density. The light scattered is measured. More particles in the wells give a higher measurement. This method is used a lot in microbiology, to count cells and bacteria growth, and is especially used for testing experiments with essential oils. Unfortunately the turbidity of the oil-water mixture can interfere with an end-point reading. (Kalemba & Kunicka, 2003, 814-816; Lawler, 2005, 343-344). 15 3.4.1 The Agar Diffusion Method The agar diffusion method is standardized for testing antibiotics’ antimicrobial activity. The method is one of the most usual ones, and has therefore also been tried for essential oils. (Kalemba & Kunicka, 2003, 814-815; Saad et. al., 2013, 270). Petri dishes are used, filled with the appropriate agar and an inoculum is spotted onto the dishes. A paper disc with a concentration of the drug is placed in the middle of the dish. After incubation, the antibiotic will have spread equally throughout the Petri dish, and a zone of growth inhibition can be visible. This zone equals the antimicrobial effect of the drug. In every dish, there is a specific concentration. (Kalemba & Kunicka, 2003, 814-815; Saad et. al., 2013, 270). The same has been tested for essential oils. A variation has also been made, where a well, or a hole, has been made in the agar and the essential oil has been distributed there. The essential oil will have trouble diffusing into the agar and spreading properly because of the volatile and hydrophobic quality. The result of the method will depend on the diameter of the paper disc or well, amount of EO, solvent used, temperature, age and concentration of the inoculum used. Therefore this technique is recommended only to use as a preliminary and pre-scanning method. (Kalemba & Kunicka, 2003, 814-815; Saad et. al., 2013, 270). 3.4.2 The Dilution Method This method has been used for fungi and bacteria. It can be done in two ways: either liquid or solid. In the agar broth method, Petri dishes are used and in the liquid broth method, tubes, conical flasks or microtiter plates are used. The inoculum and different concentrations of the antimicrobial agent is added and incubated. After incubation you can show the result in different ways, e.g. as a MIC value. It can also be presented in other ways. The different choices on how to present the result will make it difficult to compare. The microtiter method is useful since it needs only small volumes and you can use a large number of samples at once. (Kalemba & Kunicka, 2003, 816-816; Saad et.al., 2013, 270). Here follows a better description of the dilution method where a MIC value is the chosen way to present the results. 16 Minimal Inhibitory Concentration (MIC) is a numeric result used worldwide. It tells the lowest possible concentration of an antimicrobial that still prevents a visible growth. When susceptibility is examined, MIC is the standard that all new methods are compared to. MICs are used in research when developing new drugs. In clinical work, MICs are used for determining the resistance of micro-organisms. (Andrews, 2001, 5). MICs are usually made in a doubling dilution. Concentrations have to be made above and below the expected MIC. The organism incubates overnight (several nights for anaerobic microbes) and after that the MIC can be determined. MIC can be examined with two techniques; agar dilution and broth dilution (macrodilution and microdilution). It is advisable to use a control stem with a known MIC value in every assay. The MIC value you get should be plus or minus one two-fold dilution of the known MIC value. (Andrews, 2001, 10). Agar dilution means Petri dishes with different concentrations of the drug. First, a medium suitable for the organism is needed. Before pouring plates with this medium, the drug should be added, with variations in concentration. After the agar has set the plates can be kept in 4-8°C. (Andrews, 2001, 10). The inoculum should be 104 cfu/spot or 0.5 McFarland standard. The inoculum has to be distributed onto the dishes within 30 minutes of making it. Drug free control Petri dishes are necessary to include in every experiment. After incubation, which is usually 18-20 hours at 35-37°C, depending on the microbe, a MIC determination can be made. There has to be growth on the drug free Petri dish. The MIC is the same concentration that is on the dish where no visible growth can be seen. The presence of one or two colonies does not count as growth. (Andres, 2001, 10-12). Broth dilution MIC is the same thing as agar dilution, but done in a different way. Instead of Petri dishes, tubes for macrodilution or 96-well plate for microdilution are used. A suitable medium for the microbe is needed and the final inoculum should be 105 cfu/ml. (Andrews, 2001, p. 12, 16). In macrodilution, the tubes have different concentrations of the drug. In microdilution different wells have different concentrations. The tubes or wells should be mixed thoroughly before incubation. The MIC will be the tube or well with no visible growth. (Andrews, 2001, p. 12, 16). 17 3.4.3 Checkerboard Method (CB) The Checkerboard method is used to examine the synergy between two drugs. This is the most common way to examine the interaction between two antimicrobial substances. Many patients get several medications at the same time. The combination of two drugs might boost a treatment or hinder it. It is crucial to examine the interactions between two drugs to give patients the best care. (Hsieh & Yu & Yu. 1993, 343; Langeveld et.al, 2014, 81). The assay is made in tubes or with a microtiter plate. The concentrations of the drugs are usually a few steps below and above the expected MIC. Twofold concentrations are used. On one axis is drug X and on the other axis, drug Y. Both drugs have the same concentrations. Each tube or well has a different combination of the two drugs. Control columns are important; this means a column or row with only one of the drugs. (Hsies et. al, 1993, p.343-344). For the CB assay a fractional inhibitory concentration (FIC) can be calculated. The FIC is calculated for a given well, a negative well in the growth-no-growth interface. This given well is the last well where there is no visible growth. The drug concentration is divided with the control MIC of the test organism to that particular drug. The FIC is a sum of both drugs used in that particular well. (Hsies et. al, 1993, p.344). The CB method will show at which concentrations two drugs interact and eliminate the microbe. It shows a variety of potential concentration mixtures, from where the most suitable one could be investigated more. The 96-well plate allows the possibility to investigate a large amount of concentrations at one time. (Bassolé & Juliani, 2012, 39943995). There are a few problems with this assay. The twofold dilution gives an exponential curve in concentrations. A lot of concentrations are therefore “hidden”. This is an issue when it comes to drugs with a small therapeutic ratio. Another problem is that many researchers have allowed a “one-well error”, meaning that if one well is not what is expected, they can dismiss it. A third problem with CB assay is that a lot of “skipped” wells occur. This is a well that shows growth even though all the surrounding wells do not show any growth. This error is highly linked to error in technique, an artefact or a varying resistance within the strain. It is still not a desirable outcome of the assay. (Hsies et. al, 1993, p. 346-347). 18 3.4.4 Other methods Time-kill and microatmosphere method can also be used for essential oils. These methods are not being used that much. The time-kill method examines synergism between two essential oils and/or their components (EO/Cs). It measures the sub-inhibitory concentration of one of the agents to kill the other over time. When the killing ability of the first oil is enhanced by the other one, the interaction is synergetic. It is antagonistic if the effect is minimized by the sub-inhibitory concentration. (Bassolé & Juliani, 2012, 3996). Microatmosphere method is a slightly changed version of the disc diffusion method. This is better suited for essential oils that have a strong volatility. A disc is impregnated with an essential oil and added to the lid of a Petri dish. The dish with the cultivated microbe is inverted and incubated and a zone of inhibition will serve as the result. (Kalemba & Kunicka, 2003, 816). The infections caused by Candida species are often associated with biofilms and can therefore be tricky to treat. To examine the possible biofilm-destroying effect of essential oils, biofilm assays can be made. The assay is usually performed in a 96-well plate, where the inoculum first incubates and forms a biofilm. This biofilm is then challenged with essential oils or their components, with different concentrations. After incubation, XTT (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) and menadione can be used to assess the metabolic activity. XTT transforms to tetrazolium formazan from tetrazolium with active mitochondria. Menadione speeds up the process. After a short incubation, a read-out can be made, measuring the biofilm turbidimetrically. As an example, results have shown that carvacrol, geraniol and thymol could inhibit 80% of the C. albicans biofilm. (Dalleau & Cateau & Bergès & Berjeaud & Imbert, 2008, 572573). A few tests have been made on insecticides like head lice. Head lice often infect children between the ages of 3-12 and is a common parasitic infection. Resistant strains have shown up in the past few years. (Toloza et.al., 2010, 1; Gutièrrez et.al., 2015, 1). In a study by Toloza et. al. (2010, 1-3) essential oils’ effect were checked on already resistant strains of head lice. They collected lice from children 6-13 years old and tested them with 25 different essential oils. 60µl of essential oil was deposited on a micro coverglass inside a Petri dish. Inside the Petri dish, 15 head lice were put. They observed the head lice every 5 19 minutes for 60 minutes. They found differences in the effect of the essential oils. Cinnamomum prophyrium was the most effective one. 3.4.5 Growing microbes with the right medium Right conditions and media need to be used in order to grow cultures of the wanted microorganism. There is a large variety of microbes, all with their special needs. Nutrients needed for growth has to be present in the medium. Temperature, gas and pH are also important parts of a successful culture. Selective components are sometimes incorporated. This will inhibit growth of unwanted microbes. (ECACC & Sigma-Aldrich, p. 15, 18; Atlas, 2010, p. 6). Agar is a solidifying agent that be added to medium. This is used to make solid medium. There are several different agars that can be used. Many media can be bought in powder form, with instructions on how to make the medium. (ECACC & Sigma-Aldrich, p. 15, 18; Atlas, 2010, p.1). The medium contains several important aspects. Inorganic salts keep the osmotic balance of the cells. A buffering system ensures that changes to the pH won’t be affecting the culture. Carbohydrates are the main source of energy, e.g. glucose and galactose. Amino acids, and especially essential amino acids need to be in the medium, so that the cells can grow. Other elements that you can find in a medium are vitamins, proteins, peptides, fatty acids, lipids and trace elements. (ECACC & Sigma-Aldrich, p.17-18). A good medium for yeast includes peptone, yeast extract, dextrose or glucose. Yeast extract peptone dextrose (YPD) is a common routine medium for yeast. YPD can contain either dextrose or glucose. YPD can be used as in liquid or agar form. (Sigma-Aldrich). Sabouraud (SAB) is a medium used for fungi, mostly dermatophytes. SAB is a selective medium that inhibits the growth of bacteria. The pH is not suitable for bacterial growth. The medium contains peptones, glucose and agar. (Hare, 2008). Mueller-Hinton II (MH2) agar is used for susceptibility testing on bacteria. It contains beef extract, acid hydrolysate of casein, starch and agar. (Atlas, 2010, 1250). 20 Rosewell Park Institute Medium, RPMI, can be used for a large number of culture needs. It was derived from cell cultures with human leukemic cells. It is specially designed for hematopoietic cells. (ECACC & Sigma-Aldrich, p. 16) 21 4 Materials and Methods Several assays were used to confirm the effect of the essential oils or components. Minimal Inhibitory Concentration (MIC) and Checkerboard Assay (CB) were mostly used. A novel assay was also used, but that will not be discussed further in this bachelor’s thesis. A few experiments with the Biofilm Assay (BF) were made as well. All the assays were done under sterile conditions. Candida albicans was investigated the most. Candida glabrata, Staphylococcus aureus and Pseudomonas aeruginosa were also investigated. Table 1 has an overlook of the strains used. Strain Remarks Source Candida albicans SC5134 Wild type clinical isolate Gillum & Tsay & Kirsch, 1984 Candida glabrata ATCC2001 Wild type clinical isolate American Type Culture Collection (ATCC) (CBS138) Staphylococcus aureus MRSA Multi-resistant Urszula University (UG) Burn wound isolate, multi- Dr. Jean-Paul Pirnay, Military resistant Hospital Nederoverheembeek poultry AV4 Pseudomonas aeruginosa Br667 (MHN) Table 1. The microbes used in this thesis. The original C. glabrata and C. albicans strains grew on Petri dishes with YPD agar. The overnight cultures grew on Sabaroud agar Petri dishes. S. aureus and P. aeruginosa grew on Mueller-Hinton II agar Petri dishes. All of the agar dishes were made by hand in the laboratory. The protocols and materials for making these are found in appendix 2. The medium used in the assays were Rosewell Park RPMI for fungi and Mueller-Hinton II for bacteria. All of the assays were done in 96-well plates from Gilson. Polystyrene (PS) plates were used but sometimes polypropylene (PP) plates were used, in order to compare results. PS is more useful since it has clear plastic, in comparison with PP that is opaque plastic (Plastics Europe). For the read-outs, a clear 96-well plate is more beneficial, since the read-out machine reflects light. Mostly round bottomed plates were used, but in BFA flat-bottomed 22 ones were used. The plates were sterilized in BIORAD GS Gene Linker UV Chamber with UV light for 2x90 seconds. Different essential oils and components (EO/Cs) were used for the experiments. All the EOs and EOCs are coded. E1 means essential oil 1 and C1 means essential oil component 1. At the laboratory, two different essential oil libraries were in use. One numbers from 1199, the other from 200-450. E1 and E201 is the same oil, but in different libraries. E1A and E1B are the same essential oil but a different batch therefore they can have a slightly different composition. M2 and M3 are mixture of two essential oil components (EOCs). For the protection of the study there will be no information about what the different EOs and EOCs are. I have chosen to stay with the same numeration of the oils and components that were used while working instead of re-naming them “E1” and “E2” and so on. The EO/Cs used in this thesis are listed in table 2. All the EOs and components are obtained from Pranarôm International. Essential oil (E) Essential oil component (C) Mixtures (M) E3A / E3B / E203 C1B M2 E19 C2 M3 E41 E55 E98 E79 / E79A / E279 E121 Table 2. Essential oil, components and mixtures used in this thesis. If the EO/Cs were diluted, it was in dimethyl sulfoxide, DMSO (1:1). DMSO is used as a dispersing solvent and oil solubilizer. (Hili & Evans & Veness, 1997, 269-270). DMSO had its own control in most of the assays, to check if it would have any effect on its own. The mixture of EO/Cs and DMSO was done before adding it to the medium or experiment, made in Eppendorf tubes and mixed thoroughly with a vortex. Micropipettes and multichannel micropipette (Gilson) were used for the experiments. The plates were incubated at 35°C, 36°C or 37°C, and incubation time was 18-24 hours, depending on the microbe. After the incubation, the wells were mixed, if not said otherwise, with a multichannel pipette before the read-out. A read-out was made with BioTek Synergy H1 Hybrid Reader at OD600 and a scan was taken of the plate(s), where the 23 optical density of the wells are being read. Whenever read-out is mentioned in this thesis, this is the measurement that it refers to. The starting concentration was spotted onto either YPD agar or MH2 agar Petri dishes, depending on the microbe. The goal was to achieve 10-100 colonies after incubation overnight. Two samples were made of each dilution, but only two dilutions were made. The dilutions were suspended in PBS (x1) if they needed to be diluted. Counting the starting dilution with colonies helps to check if the concentration was indeed the right one. (See picture 2). Picture 2. Here is an example of how the starting concentration Petri dishes of C. albicans could look. In this experiment, I unfortunately had a miscalculation, which can be seen that the D2 dish contains 30 colonies and the D1 dish contains 315, when the goal was 10 and 100. The B means that it is the second dish of the same concentration (I always made A&B of every dilution). D0 means the original concentration, D1 means that it has been diluted 1/10 from the original and D2 means 1/100 from the original. A pin tool was used after each assay. A pin tool can transfer the wanted amount of liquid from all 96 wells at one time to the wanted surface. There are several names to this technique and tool; it can be referred to as pin tool, pinner, frogger and replicators, to mention a few. I will use the term pin tool and frogged plates when talking about the end result. (V&P Scientific). A standard 96-well frogger (brand unknown) was used for the assays. An example of how the pin tool looked can be found in picture 3. The pin tool was dipped in the 96-well plate, stirred around a little bit and afterwards pinned onto square agar plates, with either YPD agar for fungi or MH2 agar for bacteria. Between every use of the pinner, it was dipped in 70% ethanol and put under a flame, to sterilize it. Dilutions of the 96-well plate was sometimes made. 20µl of the original 96-well plate was mixed with 24 180µl of PBS (x1) in a new 96-well plate. This was made as many times as desirable. The original 96-well plate was called D0 and the next one D1 and so on. The pinned square plates were incubated at 35-37°C for around 18 hours and scanned. Picture 3. An illustration of a how a manual pin tool can look like. The frogging technique is used to assess if the essential oils had a fungicidal (or bactericidal) or fungi static (or bactericidal) effect on the microbes. If there are no growth the effect is fungicidal (killing off the microbe) and if there are a few microbes visibile it is fungi static (inhibiting). Sometimes there is a high measurement from the read-out machine in wells with high concentrations of the turbid EO/Cs. If this is the case, you can prove it with the frogging technique, since no growth is seen on the square agar plate. A variation of these methods was made, using a kinetic assay. In this kind of assay, a similar plate is prepared, but only one can be measured at a time. The assay takes around 60 hours to run and it measures the growth of the microbe at different time points. The experiment was analysed with Bio-Tek Synergy H1 Hybrid Reader at OD600. The plate is constantly shaken and kept at the right temperature. Thanks to the kinetic assay you get a better view on how the essential oils affect the growth over a longer period of time. A continuous measurement is made instead of an endpoint measurement. With the help of the kinetic assay you can also determine which concentration of EOs would have the most effect and be able to optimise the assays in use. 25 4.1 Minimal Inhibitory Concentration (MIC) The MIC of C. albicans was examined, with microdilution as the main method. The strain of C. albicans grew overnight at 37°C on a Sabouraud agar Petri dish. A few cells were suspended in 1 ml PBS (x1). A 10-2 dilution was made in PBS (x1). The cells were counted with a spectrophotometer and adjusted to 5x103 cells/ml in RPMI, using the formula OD1 = 107 cells/ml. Due to a mistake in calculating, all the MIC assays were done with a final concentration of 2,5*104 cells/ml and not the intended 5*103 cells/ml. The results can still be interpreted, but not used in any scientific article, due to the miscalculation. They are shown here since they still show antimicrobial effect. The starting concentration was spotted onto YPD Petri dishes and incubated at 37°C overnight. Six different EO/Cs were used in one assay. The EO/Cs were mixed with DMSO (1:1). The concentration of EO/Cs was 1%, 0.5% and 0.25%, diluted in RPMI to a total volume of 100µl for each well. Each concentration was pipetted twice in the area with cells. In order to see the effect of EO/Cs, they were pipetted in the same concentration in the wells with no cells. In the control column, the same concentrations were made, but only with DMSO, no EO/C, to see the effect of DMSO. (See table 3) PBS 1 PBS A EO/C B EO/C C EO/C D EO/C E EO/C F EO/C PBS G H 1% EO/C 2 1% EO/C 3 0.5% EO/C 4 0.5% EO/C 5 0.25% EO/C 6 0.25% EO/C 7 DMSO 8 1% EO/C 9 0.5% EO/C 10 0.25% EO/C 11 1% DMSO 1% DMSO 0.5% DMSO 0.5% DMSO 0.25% DMSO 0.25% DMSO Table 3. MIC Assay Chart. Grey area is PBS (x1). Green areas had cells, red had not. Column 8 has no EO/Cs. Row B-G all have different EO/Cs. The final volume in all wells is 200µl. PBS 12 26 The experiment was carried out so that 200µl PBS (x1) was distributed in the outermost wells. This will act as a control and as a hydration to the other wells. 100µl inoculum or RPMI was put in the rest of the wells, according to table 3. After that, the right amount of RPMI was pipetted, followed by the mixture of EO/C and DMSO. In the column 8, no EO/Cs were used, the concentration there is the DMSO one. In the area without any cells, the concentration is meant to be EO/Cs mixed with DMSO. After pipetting, the wells were mixed together carefully with a multichannel pipette. The plate(s) were incubated at 37°C for 18-24 hours. After the incubation the wells were shaken with a multichannel pipette. A read-out, scan and the pinning technique was applied. Four experiments were carried out. In the first experiment, four different variations were made; PP plate where the EO/Cs were mixed with DMSO and one without DMSO, PP plate where the EO/Cs were mixed with DMSO and one without DMSO. In the following three experiments only the variation PS+DMSO was used. With or without DMSO indicates if the EO/C was first mixed in DMSO (1:1) or not. Only the second and the fourth experiment are shown in results. In the first experiment C1B, C2, E3A, E19, E121 and E279 were used, in that order. The plates were incubated for 24 hours at 37°C. The read-out was made with both unshaken wells and shaken wells. The 37°C were after this optimised to 35°C. In the second experiment C1B, C2, E3A, M2, M3 and E79 were investigated. M2 and M3 are mixtures of different essential oil components that were made in the laboratory. A PS plate was used and the EO/Cs were mixed with DMSO (1:1). Incubation was at 35°C for 18 hours. The third experiment was a kinetic assay. C2, E19, E41, E55, E98 and E121 were used, all mixed with DMSO (1:1). A PS plate was used and the read-out machine incubated the plate at 37°C for about 60 hours. The fourth experiment was made to check essential oils for the checkerboard assay. The EO/Cs used were E3A, E3B, E203, E79, E79A and E279. A PS plate was used and EO/Cs were mixed with DMSO (1:1). The plate was incubated at 35°C for 24 hours. 27 4.2 Checkerboard Assay (CB) Four microbes were examined with the CB assay; Candida albicans, Candida glabrata, Staphylococcus aureus and Pseudomonas aeruginosa. C2s interaction with E79, C1B and E279 were examined. C. albicans and C. glabrata were first grown overnight at 37°C on Sabouraud Petri dishes. A few cells were picked up and put in 1 ml PBS(x1). A 10-2 dilution was made. The cells were counted with a spectrophotometer and adjusted to 5 x 103 cells/ml (OD1 = 107 cells/ml). This dilution was made in RPMI. S. aureus and P. aeruginosa grew overnight at 35°C in tubes containing 10 ml MuellerHinton II broth. A dilution was made from this, counted with the spectrophotometer and adjusted to 5 x 105 cfu/ml. (S. aureus: OD1 = 109 cells/ml and P. aeruginosa: OD1 = 5 x 108 cells/ml). The inoculum was made in Mueller-Hinton II. Polystyrene 96-well plates were used. Rows B to G have the same EO/C, but in different concentrations. Columns 2 to 11 have another EO/C, also in different concentrations. This will make every well have a unique concentration of the two EO/Cs. One row or column was always made with 0% concentration, to get a control of only one of the EO/Cs. (See table 4). In the beginning, table 4 was used just as described. But later on, the last column (11) was utilized for only 100µl RPMI, no EO/Cs. This was due to the fact that the dilution of EO/C+DMSO made was just enough, and did not always give the exact amount in the last well. After a few experiments it got changed and column 11 contained a DMSO control. In column 11, there were different concentrations of DMSO without EO/Cs. In well B11 was 2% DMSO, in C11 1% and so on until well G11 where there was 0.06% DMSO. The DMSO row was good since it gave us an idea how only DMSO affects the experiment. 28 PBS EO/C Y 1% EO/C Y 0.5% EO/C Y 0.25% EO/C Y 0.125 % EO/C Y 0.06% EO/C Y 0% PBS PB S EO/ CX 1% 1 2 EO/ CX 0.5 % 3 EO/ CX 0.25 % 4 EO/C X 0.125 % 5 EO/ CX 0.06 % 6 EO/ CX 0.03 % 7 EO/C X 0.015 % 8 EO/C X 0.006 % 9 EO/C X 0.003 % 10 EO/ CX 0% PB S 11 12 A B C D E F G H Table 4. The model for the checkerboard assay. 96-well plate. The white areas contain inoculum and EO/C X was pipetted on one side and EO/C Y on the other. The grey areas were filled with 200µl PBS(x1). The outermost wells were filled with 200µl PBS(x1). 100µl inoculum was distributed in the rest of the wells. The EO/Cs were all mixed with DMSO (1:1). The concentration gradient was made with a serial dilution in Eppendorf tubes. Each of the tubes were mixed thoroughly. After the serial dilution, 50µl of one of the EO/Cs were first pipetted in the well, after that 50µl of the other EO/C. In the end, all of the wells were mixed thoroughly together with a multichannel pipette. Incubation lasted 18-24 hours in 35 or 37°C. A starting concentration was spotted onto Petri dishes with YPD agar for the fungi or MH2 agar for the bacteria. The next day, a read-out and a scan were made on the CB plate(s) and the pinning technique was used. Altogether 14 CB assays were made. Throughout all of these experiments, an optimization of the assay was made. All of the experiments will be shown in table 5, but results will only be given for a few chosen experiments, with the right conditions and good results. Non-optimal conditions and bad results are also discussed in the results. 29 The first assay that will be presented was made with Candida albicans. Two PS plates were made, one with C2 and C1B and another with C2 and E79. All of the concentrations started at 0.5%. The incubation time was 24 hours at 35°C. After the incubation a read-out and a scan were made and the pin tool was used. The second assay to be shown was also with two plates. The EO/Cs used were C2 and E79, both starting at 1 %. One plate had C. albicans and the other C. glabrata. Incubation was at 35°C for 24 hours. This was the first time the DMSO column was used. Before and after the incubation, a read-out was made in order to see the turbid effect of the EO/Cs. After the incubation a scan was made and the pin tool used. The third assay was a kinetic assay. This was made with C. glabrata. C2 and E79 were used, both starting at 1 %. Column 11 was a DMSO control and the assay was incubated and read continuously at 35°C for 67 hours. The last assay that is mentioned is of S. aureus and P. aeruginosa, on two different plates. The EO/Cs used were C2 and E79, both starting at 1 % concentration. Incubation was 18 hours, at 36°C. There was a DMSO column. 30 Experiment Number Microbe Incubation Time (time-order) 1 C. albicans 18h EO/C X EO/C Y - starting concentration - starting concentration C2 – 0.5 % C1B – 0.5 % Column 11 - E79 – 0.5 % 2 C. albicans 24h C2 – 0.5 % C1B – 0.5 % - E79 – 0.5 % 3 C. albicans Kinetic Assay C2 – 0.5 % C1B - 0.5 % - 4 C. albicans 24h C2 – 1 % C1B – 0.125 % - E79 – 1 % 5 C. glabrata 24h C2 – 1 % C1B – 0.125 % - 6 C. albicans 24h C2 – 1 % E79 – 1 % RPMI E279 – 1 % 7 C. albicans Kinetic Assay C2 – 1 % E79 – 1 % RPMI 8 C. alb / C. gla 24h C2 – 1 % E79 – 1 % DMSO 9 C. glabrata Kinetic Assay C2 – 1 % E79 – 1 % DMSO 10 S. aur / P. aer 18h C2 – 1 % E79 -1 % DMSO 11 S. aur / P. aer 18h C2 – 1 % E79 – 1 % DMSO 12 S. aur Kin C2 – 1 % E79 – 1 % DMSO 13 S. aur 16h C2 – 1 % E79 – 1 % DMSO 14 P. aer Kinetic assay C2 – 1 % E79 – 1 % DMSO Table 5. An overview on all the CB assays made. This shows the order of the experiment in the time-lapse they were made, not with any other significance. The EO/C X starting concentration shows the first EO/C starting concentration and the same thing goes for EO/C Y. Sometimes multiple plates were made at the same time, for example C. alb / C. gla means that the assay was made twice, on two different plates, with two different fungi. Two EO/Cs means that the same experiment was made but with two different plates and with different EO/Cs on the Y axis. 31 5 Results The results from two MIC assays and four CB experiments are presented. These results are all valid and give good information about the investigation. The endpoint measurement and a frogged result are shown for all the experiments. If all the values in the outermost wells are similar, it means that the control is acceptable. PBS (x1) should not have any growth. Usually the value is around 0.08-0.1. The read-out machine gives different colours depending on the values received in the measurement. Two plates are not comparable to each other, according to colour only. Where there is a higher value, and a darker colour, there are more particles in that well. This likely means that there is growth in those wells. According to this result, you can make some assumptions of the result, but the whole truth is not visible here. 5.1 Minimal Inhibitory Concentration All of the MIC assays were made with Candida albicans. The EO/Cs used are C1B, C2, E3A, M2, M3 and E79. PBS A PBS 1 0.115 1% 2 0.087 1% 3 0.092 0.5% 4 0.088 0.5% 5 0.12 0.25% 6 0.102 0.25% 7 0.083 DMSO 8 0.092 1% 9 0.087 0.5% 10 0.09 0.25% 11 0.09 PBS 12 0.102 C1B C2 B C 0.094 0.094 0.094 0.178 0.096 0.099 0.106 0.149 0.096 0.159 0.285 0.111 0.095 0.118 0.479 0.426 0.098 0.145 0.094 0.102 0.1 0.101 0.093 0.124 E3A M2 D E 0.092 0.084 0.093 0.094 0.098 0.113 0.611 0.11 0.746 0.102 0.627 0.096 0.593 0.098 0.427 0.586 0.11 0.126 0.105 0.107 0.116 0.09 0.083 0.087 M3 E79 F G 0.085 0.134 0.103 0.265 0.09 0.368 0.102 0.242 0.099 0.207 0.097 0.269 0.093 0.556 0.332 0.437 0.092 0.296 0.094 0.178 0.093 0.15 0.104 0.082 PBS H 0.095 0.081 0.09 0.083 0.084 0.098 0.1 0.089 0.082 0.085 0.081 0.108 Table 6. Read-out result for a MIC-assay with C. albicans. The outermost wells are PBS (x1). On the X axis you can see what EOs were used (C1B, C2, E3A, M2, M3, E79). Y axis shows the concentration of the EO. Column 8 is a DMSO control. Column 9, 10 and 11 do not contain the fungus. The controls look good for this experiment. A contamination has occurred in only one of the wells (G1). Column 8 has growth since there is only inoculum and DMOS there. For the interpretation you can say that C1B shows inhibition of growth, but there is a well (B6) that does not seem to correlate with the results. C2 has an inconclusive row. For E3A it 32 seems that the MIC is 1%. For M2 and M3, all growth have been inhibited. E79 seems to have some kind of inhibition, but give different values for the 0.25%, which is not expected. Higher values are also on the side with no cells (G9 & G10). Picture 4. Result with pin tool on MIC assay with C. albicans. EOs used were C1B, C2, E3A, M2, M3 and E79. The concentrations of the EOs are visible on top. Also the DMSO column and the area without inoculum are shown. By looking at the frogged plate (picture 4), you see a difference in the result. You can see growth in all of the DMSO wells. In this picture, no growth in the controls or in the wells without cells are visible. This means that the controls are okay, and the read-out’s measurements has reacted on something else. Comparing to the read-out result, the E79 oil changes the turbidity drastically, and the value in those wells have a high measurement, but here there is actually no growth on the side without cells. The MIC value of E79 will be 0.5%, as there is growth in the 0.25% wells. C1B inhibited all growth, and so did M2 and M3. The well that had a high read-out (B6) is not indicated at all here, so that will count for as an error. C2 gave a peculiar result, as one of the 0.25 % wells has no growth, while the other had. E3A has growth in 0.25 % and 0.5 %, so its MIC value will be 1%. The other MIC experiment examines E3A, E3B, E203, E79, E79A and E279. Since the three first essential oils are the same, with yet slightly different composition, and the same goes for the three last ones, similar results are to be expected from these groups. This MIC 33 assay was also made in order to find an essential oil that would not have a significant antimicrobial effect. PBS E3A E3B E203 E79 E79A E279 PBS A B C D E F G H PBS 1 0.084 0.082 0.085 0.085 0.084 0.08 0.085 0.097 1% 2 0.084 0.089 0.279 0.265 0.162 0.101 0.092 0.118 1% 3 0.087 0.097 0.387 0.392 0.418 0.098 0.101 0.084 0.50% 4 0.087 0.093 0.421 0.391 0.309 0.097 0.098 0.085 0.50% 5 0.089 0.097 0.374 0.368 0.257 0.105 0.098 0.084 0.25% 6 0.086 0.102 0.383 0.382 0.389 0.171 0.157 0.084 0.25% 7 0.089 0.095 0.375 0.366 0.189 0.099 0.209 0.088 DMSO 8 0.085 0.366 0.363 0.39 0.455 0.447 0.448 0.088 1% 9 0.085 0.095 0.105 0.099 0.334 0.097 0.099 0.085 0.50% 10 0.085 0.094 0.096 0.099 0.288 0.104 0.1 0.086 0.25% 11 0.085 0.093 0.089 0.135 0.124 0.096 0.095 0.086 Table 7. MIC result of C. albicans. The outermost wells are PBS (x1) controls. On the X axis you can see what EOs were used (E3A, E3B, E203, E79, E79A, E279). Y axis shows the concentration of the EO. Column 8 is a DMSO control. Column 9, 10 and 11 do not contain the fungus. The result of this assay is more interesting (table 7). The controls look good. There is growth in every well of the DMSO column. Here a slight impact on the concentration of DMSO is seen. There seems to be more growth where there is less DMSO. It indicates that DMSO could have a minor antimicrobial effect. On the area without cells, there are some higher values linked with E79 and one well with E203. E3A seems to inhibit all growth. But E3B and E203 seem to have had only little effect on the growth at 1%. There seems to be some growth in the E79 row, but in a quite illogical way. E79A and E279 have similar results. When comparing this result to the other MIC result, you can see that the E3As MIC value was 1%, when here the oil inhibits all growth. PBS 12 0.083 0.083 0.082 0.083 0.089 0.088 0.086 0.085 34 Picture 5. Pinned result of C. albicans. MIC assay with EOs E3A, E3B, E203, E79, E79A and E297. This is made from the original plate, i.e. D0. The concentrations of the EOs are visible on top. Also the DMSO column and the area without inoculum are shown. Pinned results were made with D0 and D1 (picture 5). E3A inhibits all growth, just as on the read-out. E3B and E203 seem to have no effect with these concentrations, and grow on a full scale. E79 inhibits all growth while E79A has a MIC of 0.5%. With E297 it is hard to tell if the MIC value would be 1% or 0.5%, since there is an irregular pattern in the wells. This frogged plate is a little bit messy, with some drops beside the wells. No growth is shown on the side with no cells, which means that the read-out had high measurements due to E79’s oil. These results were not as expected, since E3A, E3B and E203 should have had similar results, as with E79, E79A and E279. To show how the 96-well plate could look like, there is a scanned picture of the plate (picture 6). A scan was always made to be able to compare the read-out machine’s result and the pinned result with the way the wells looked visually. You can see where there is growth and not, and where the oil-water emulsion mixture is visible (looks like growth). 35 Picture 6. This is how the 96-well plate looked after the incubation. You can clearly see where there is growth and where there is no growth. Row E (E79) has a lot of turbidity even though there is no growth. 36 5.2 Checkerboard Assay The checkerboard assay was made with all four microbes. Assays with the Candida species, a kinetic assay with C. glabrata and an assay with S. aureus and P. aeruginosa are presented. All of the assays examined the essential oil component C2’s interaction with either C1B or E79. 5.2.1 Candida albicans with C2 and C1B or E79 (CB assay) The first experiment was made with C. albicans, incubated for 24 hours. Two plates were made, one with C2+C1B and the other with C2+E79. The starting concentrations are 0.5% for all the EO/Cs. PBS 0.50% 0.25% 0.125% 0.06% 0.03% 0% PBS A B C D E F G H PBS 1 0.081 0.081 0.082 0.081 0.082 0.083 0.084 0.085 0.5% 2 0.083 0.143 0.111 0.102 0.096 0.103 0.098 0.083 0.25% 3 0.083 0.136 0.106 0.103 0.099 0.134 0.249 0.086 0.125% 4 0.084 0.141 0.098 0.2 0.383 0.325 0.329 0.084 0.06% 5 0.082 0.128 0.154 0.409 0.34 0.302 0.326 0.087 0.03% 6 0.084 0.139 0.365 0.452 0.418 0.342 0.233 0.092 0.015% 7 0.084 0.114 0.443 0.468 0.402 0.324 0.23 0.088 0.006% 8 0.084 0.122 0.442 0.403 0.33 0.31 0.237 0.087 0.003% 9 0.083 0.203 0.451 0.374 0.401 0.305 0.205 0.085 0.0015% 10 0.081 0.255 0.21 0.177 0.181 0.184 0.168 0.087 0% 11 0.082 0.291 0.189 0.154 0.157 0.188 0.192 0.082 PBS 12 0.081 0.081 0.082 0.081 0.081 0.082 0.087 0.082 Table 8. CB assay with C. albicans, incubation 24 hours. The 96-well plate has a checkerboard look to it, which symbolises the interaction between the two EO/Cs. C2 on X axis and E79 on Y axis. Concentrations used are also shown. The outermost wells contain PBS (x1). When you look at the result (table 8), there is a clear checkerboard structure of the measurements. Controls look good and there is nothing special in row 11. In the 0 % row or column you can observe the effect of only one of the EO/Cs. 0.5 % C2 kills of all the growth in column 2. E79 is not as strong, but still kills of a lot of the growth in row B. This is comparable with C1B instead of E79. 37 PBS 0.5% 0.25% 0.125% 0.06% 0.03% 0% PBS A B C D E F G H PBS 1 0.086 0.085 0.087 0.083 0.084 0.086 0.083 0.085 0.5% 2 0.083 0.091 0.093 0.095 0.094 0.089 0.094 0.085 0.25% 3 0.083 0.092 0.097 0.094 0.095 0.091 0.266 0.079 0.125% 4 0.085 0.093 0.094 0.093 0.097 0.158 0.318 0.088 0.06% 5 0.086 0.09 0.094 0.093 0.096 0.27 0.257 0.086 0.03% 6 0.086 0.091 0.091 0.09 0.096 0.276 0.253 0.086 0.015% 7 0.085 0.092 0.093 0.093 0.091 0.244 0.198 0.086 0.006% 8 0.086 0.092 0.092 0.092 0.093 0.233 0.224 0.107 0.003% 9 0.083 0.099 0.092 0.092 0.094 0.232 0.245 0.086 0.0015% 10 0.084 0.207 0.168 0.172 0.165 0.174 0.191 0.088 0% 11 0.082 0.198 0.192 0.17 0.169 0.172 0.236 0.09 PBS 12 0.081 0.087 0.086 0.085 0.086 0.088 0.087 0.084 Table 9. CB assay with C. albicans, 24 hour incubation. C2 on X axis and C1B on Y axis. Concentrations used are also shown. The outermost wells contain PBS (x1). A lot of the growth is killed off in this experiment. The interaction between C2 and C1B (table 9) is greater than the interaction between C2 and E79 (table 8). The components are stronger together and a smaller concentration of them still manages to kill off more growth than the combination. Since there is the same C2 on both assays, concludes that it is the C1B which gives the difference. In future assays the concentration of C1B was lowered, in order to get a more useable result, since a lot of the wells have no growth. It is better to investigate if you find that line where the inhibition works, and a few concentrations below and above that. Since E79 and C2 seem to kill off only one row, that concentration was lowered to start at 1 %. 5.2.2. C. albicans and C. glabrata with C2 and E79 (CB assay) C. albicans and C. glabrata were both investigated with the interaction between C2 and E79. The concentrations of both of the EO/Cs started at 1 %. In column 11, a DMSO control column was incorporated. A scan was made before and after the incubation to compare the values with the measurement of the sample (picture 6). You can clearly see that the EO/Cs alone give a strong read-out. This is why it is so important to also do the frogging technique. 38 Picture 6. Here you can see a quick overlook on the plates before the incubation. The first table (C. albicans) has high read-out values before the incubation. These values are due to the strong viscosity of E79. The effect is also seen on the second table with C. glabrata. Apparently E79 gives a stronger viscosity with C. albicans, and lower with C. glabrata. The result for Candida albicans (table 10) looks good. The controls are okay. There seems to be high values in the high concentrations of the EO/Cs, but that is because of the consistency of the oils. DMSO has a consistent row, with less growth along with less concentration. This is the opposite of what was seen before, here it seems that more DMSO gives a little bit more growth. The well G10 is the growth control. In that well there are no essential oils, 0% of both of them. This is interesting because it seems as though the essential oils enhances the growth at lower levels of the EO/Cs. C2 seems to inhibit the first column completely and the same goes for E79. Looking at the wells with only a percentage of one of the EO/Cs, you see that E79 inhibits growth until 0.5% (row 10). C2 seems to only inhibit at 1% on its own (row G). PBS 1% 0.50% 0.25% 0.125% 0.06% 0% PBS C. alb A B C D E F G H PBS 1% 0.50% 0.25% 0.125% 0.06% 0.03% 0.015% 0.006% 0% DMSO PBS 1 2 3 4 5 6 7 8 9 10 11 12 0.081 0.083 0.082 0.082 0.084 0.079 0.092 0.082 0.081 0.267 0.22 0.164 0.113 0.103 0.091 0.082 0.082 0.2 0.205 0.126 0.118 0.384 0.336 0.081 0.081 0.303 0.243 0.147 0.387 0.398 0.273 0.081 0.081 0.238 0.166 0.248 0.395 0.362 0.279 0.081 0.082 0.247 0.222 0.303 0.388 0.395 0.27 0.083 0.082 0.272 0.224 0.373 0.399 0.389 0.26 0.082 0.081 0.294 0.222 0.434 0.401 0.389 0.229 0.081 0.08 0.195 0.136 0.41 0.414 0.369 0.26 0.081 0.082 0.192 0.2 0.444 0.407 0.369 0.225 0.083 0.082 0.323 0.304 0.298 0.279 0.247 0.251 0.082 0.081 0.081 0.082 0.086 0.087 0.08 0.102 0.082 Table 10. CB assay with C. albicans. C2 on the X axis and E79 on the Y axis. You can also see which concentrations were used. The outermost wells contain PBS (x1). Even the higher concentrations give a high read-out, due to E79’s viscosity. 39 The result on Candida glabrata is quite similar, with a few differences. (See table 11). The controls are okay and the DMSO row has equal amount of growth. There is a sharp line between the growth and no-growth zone. High values are also found in the left upper corner, but these are due to the viscosity of the EO/Cs. Here, the G10 well, is similar to the other wells, so no “extra” growth for the fungi with low levels of EOs. For the EO/Cs alone, you can see the same values as in C. albicans. E79 inhibits growth until 0.5% and C2 only inhibits at 1% (column 10 and row G). PBS PBS 1% 0.50% 0.25% 0.125% 0.06% 0% PBS C. gla A B C D E F G H 1% 0.50% 0.25% 0.125% 0.06% 0.03% 0.015% 0.006% 0% DMSO PBS 1 2 3 4 5 6 7 8 9 10 11 12 0.083 0.082 0.082 0.081 0.103 0.086 0.083 0.082 0.081 0.364 0.294 0.185 0.118 0.094 0.103 0.084 0.083 0.317 0.198 0.157 0.193 0.554 0.652 0.082 0.081 0.248 0.183 0.147 0.601 0.639 0.657 0.083 0.085 0.34 0.314 0.161 0.646 0.657 0.673 0.082 0.082 0.319 0.177 0.371 0.65 0.678 0.681 0.088 0.083 0.275 0.238 0.343 0.66 0.679 0.699 0.091 0.081 0.237 0.14 0.564 0.633 0.672 0.692 0.083 0.087 0.388 0.187 0.639 0.664 0.68 0.691 0.086 0.082 0.278 0.168 0.639 0.659 0.678 0.677 0.082 0.082 0.651 0.647 0.67 0.702 0.672 0.684 0.082 0.087 0.081 0.091 0.093 0.08 0.084 0.082 0.082 Table 11. CB assay on C. glabrata. C2 on the X axis and E79 on the Y axis. Concentrations used are shown. The outermost wells contain PBS (x1). Even the higher concentrations give a high read-out, due to E79’s viscosity. C. glabrata usually forms bigger colonies therefore D0 of C. albicans and D1 of C. glabrata are shown. On the plate with C. albicans (picture 8), you can see that C2 kills almost all growth in the first column, except the last one, where there are a few colonies visible. That means that C2 cannot inhibit the growth all on its own, at 1%. E79, on the other hand, kills all the growth in the row with 1% concentration. There is a contamination in the right corner, probably due to the wrong pinning technique. 40 Picture 8. The frogged result from CB assay with C. albicans. On top there is C2 and the concentrations used. On the right there is E79 EO and the concentrations used. All the numbers are in procent. For the pinned result with C. glabrata, it is almost the same result. (See picture 9). C2 kills everything with 1%, except for the one where there is no E79 involved. E79 kills the complete first row. A nice checkerboard assay is visible. Picture 9. The frogged result from CB assay with C. glabrata. On top there is C2 and the concentrations. On the right there is E79 and the concentrations used. All the numbers are in procent. This is D1 (diluted 1/10 of the original plate). C. glabrata forms bigger colonies than C. albicans, therefore you can see almost no difference in D0 of C. albicans and D1 of C. glabrata. 41 The reason why dilutions of the plates were made before they were pinned, was to see that the growth actually diminishes, colony by colony. In picture 10 you can see a quick overview on how it can look. Picture 10. C. glabrata pinned results after CB assay. This illustrates the differences between D0 and D3. D0, the original plate (upper left corner), D1 (upper right corner), D2 (lower left corner) and D3 (lower right corner). You can clearly see how the growth disappears, sometimes with one colony at a time. 42 5.2.3 C. glabrata with C2 and E79 (CB, kinetic assay) The kinetic assay was made in the exact same way as the rest of the assays, with the only difference that the incubation is longer and there is a continuous reading of the wells. This gives a different kind of result, but a valuable one. This experiment was made with C. glabrata. C2 and E79 were the EO/Cs of choice, and the concentration started at 1 %. Picture 11. Kinetic assay with C. glabrata. The red lines are the growth curves for each well on the 96-well plate. In the controls (outermost wells) the line is straight, which means no growth. C2 EOC on X axis and E79 EO on Y axis. The first picture shows curves of the fungal growth over around 60 hours. (See picture 11). The controls look good because the line seems to be consistent, which means that the measurements have been the same during the whole time. Row 11 is the DMSO control. The results here are pretty similar to each other. If you look at F2, you can see that the EO/Cs have completely inhibited all growth (C2 1% and E79 0.06%). The curve is exactly the same as the control ones. All of the growth curves start at the same point, except for when there are high concentrations of EO/Cs. The whole row B can be counted in here. None of the curves start at the same line as the rest. This is also portrayed in picture 12. 43 Picture 12. Kinetic assay with C. glabrata, well B2 and F2. Notice the huge difference between a no growth well (F2, red line) with the well B2 (blue line), which have the highest concentrations of both EO/Cs (1% C2 and 1% E79). The interesting thing about this assay is that you can clearly see where the essential oil or component inhibit the growth. The lag phase is extended. The difference is very big and it clearly shows that essential oils do have an effect on fungi. (See picture 13). Picture 13. Kinetic assay, C. glabrata, well G2 (red line) and G1 (blue line). The difference between G2 and G10 is very noticeable. G2 is the well with 1% C2 and 0% E79. The lag phase is extended compared to G10, where there is 0% C2 and 0% E79. 44 5.2.4 S. aureus and P. aeruginosa with C2 and E79 (CB assay) I have chosen to include results for S. aureus and P. aeruginosa in this thesis because these pathogens are of great importance to clinical microbiology. Unfortunately, good results were not received and there were no time to optimise the technique. The assay was repeated a few times and kinetic assays were also made on both bacteria, but fell short with an explanation of why the results were so out of line. I still wish to present the result here. You can see the results in tables 12 and 13, with complementary comments. PBS 1% 0.50% 0.25% 0.125% 0.06% 0% PBS S.aur A B C D E F G H PBS 1 0.085 0.096 0.089 0.096 0.093 0.094 0.086 0.081 1% 2 0.093 1.005 0.956 1.591 1.724 1.604 1.468 0.096 0.50% 3 0.097 0.922 1.281 1.701 1.731 1.72 1.571 0.094 0.25% 4 0.095 0.956 1.311 1.711 1.764 1.787 1.559 0.091 0.125% 5 0.098 1.047 1.269 1.725 1.785 1.798 1.564 0.091 0.06% 6 0.095 1.046 1.479 1.692 1.796 1.829 1.546 0.091 0.03% 7 0.095 0.995 1.395 1.705 1.834 1.822 1.579 0.09 0.015% 8 0.094 0.976 1.462 1.747 1.811 1.848 1.557 0.088 0.006% 9 0.094 0.96 1.504 1.712 1.794 1.864 1.608 0.084 0% 10 0.094 0.996 1.481 1.802 1.852 1.872 1.596 0.084 DMSO 11 0.093 1.465 1.49 1.522 1.525 1.529 1.579 0.082 PBS 12 0.09 0.093 0.098 0.093 0.098 0.097 0.09 0.083 Table 12. CB assay, S. aureus, 18 hour incubation. C2 on X axis and E79 on Y axis. The concentrations are shown. Column 11 is a DMSO control. The outermost wells are filled with PBS (x1). Growth is visible everywhere. Controls are okay. On both of the plates there are abnormally high values. The starting concentration was checked, along with other factors that might have caused this abnormal result. Careless sterile technique or the multi-resistant trait of the microbes could also have influenced the results. The controls look okay in both plates. In the plate with P. aeruginosa there seems to be some inhibition in the first and second row. There is no need to look at the pinned plates, since they look exactly as the tables shown here. Growth is almost everywhere. P.aer PBS 1 1% 2 0.50% 3 0.25% 4 0.125% 5 0.06% 6 0.03% 7 0.015% 8 0.006% 9 0% 10 DMSO 11 PBS 12 PBS A 0.084 0.093 0.092 0.095 0.097 0.091 0.095 0.093 0.092 0.091 0.091 0.084 1% B 0.091 0.887 0.518 0.516 0.675 0.772 0.782 0.737 0.632 0.422 1.47 0.089 0.50% C 0.093 0.904 1.295 0.376 1.347 0.536 1.504 0.46 1.305 1.365 1.545 0.095 0.25% D 0.093 1.354 1.417 1.454 1.498 1.487 1.541 1.588 1.516 1.639 1.571 0.097 0.125% E 0.085 1.442 1.509 1.717 1.707 1.725 1.721 1.741 1.768 1.772 1.596 0.1 0.06% F 0.089 1.51 1.625 1.859 1.847 1.833 1.881 1.849 1.856 1.892 1.63 0.086 0% G 0.084 1.543 1.606 1.647 1.622 1.64 1.634 1.654 1.626 1.621 1.664 0.08 PBS H 0.084 0.082 0.082 0.079 0.08 0.08 0.083 0.081 0.081 0.084 0.084 0.082 Table 13. CB assay, P. aeruginosa, 18 hour incubation. C2 on the X axis and E79 on the Y axis. The concentrations are shown. Column 11 is a DMSO control. The outermost wells are filled with PBS (x1). Growth is visible in the whole plate, inhibition in a few wells in row B. Controls are okay. 45 6 Discussion and Conclusion In this thesis different assays have been evaluated and compared in order to assess the antimicrobial activity of essential oils and their components. In most of the experiment made, some kind of antimicrobial activity is visible. Unfortunately, the assays used in this thesis are not standardized for antimicrobial testing with essential oils. This led to that the results were not optimal or the same as what was expected. The fact that the oils might have been derived differently, will have an effect too. DMSO was the solvent best fit for this assay. By studying the results, the DMSO used seem to have inhibited the growth sometimes and enhance it sometimes. It would have been good to try which solvent would have had the least effect on the assay before starting the investigation. Another solvent might have worked better. Sterile conditions were used during all of the experiments. A fault in this technique could also explain why DMSO gave such different results. If the DMSO solution is not solvent, it will give false results on the assay. It is questionable if DMSO could be used to dissolve the EO/Cs in a medication. Investigating the EO/Cs together with the right solvent for human beings, is an important part that needs to be taken into consideration. Luckily, it’s clearly visible that essential oils and their components do have an antimicrobial activity. The growth is inhibited to some extent in every assay, though the scale of essential oils used in this thesis was small. C2 and E79 clearly seem to have antimicrobial effects, as well as having a good interaction between each other. It is unfortunate that my experiments cannot be reproduced, since I have no record of what these essential oil and essential oil components actually are. The work could have been optimised a little bit more. The CB assays were the most successful ones, with an exception of the ones made with S. aureus and P. aeruginosa. The CB technique is a tricky technique and requires a long time of practising. Switching microbes towards the end gave poor results. Optimising this would be the next step. The bacteria strains were both multi-resistant, which could also explain the big difference between the CB assays for fungi and those for bacteria. The essential oils inhibit the growth at an enough high concentration but can sometimes enhance the growth, if the concentration was a little bit too small to kill it off. This was an interesting result, which was seen during multiple experiments. 46 It seems as though essential oils have cytotoxic properties but are also capable of giving nutrients or enhancing growth. It is an interesting find, and the research definitely needs to focus on mapping toxicity of essential oils. It is important to remember that essential oils work on both eukaryotic and prokaryotic cells. This means that we need to figure out what effects the oils can have on our own cells. It is necessary to figure out if there is any use in investigating essential oils if they will do harm to our body. Developing a standardized way of extracting and examining essential oils is crucial. Even in this research it is visible that oils that are meant to be the same can have different results, just because they are derived from another point and place. This is most definitely a problem in the future, unless a standardized way of developing the oils is invented. In my experiments, E79 was effective, in comparison to E279, which is the same essential oil. This clearly lowers the quality and reproducibility of the experiments. Essential oils are not the easiest to work with due to their volatile and lipophilic character. It was often hard to blend the oil together with the medium and this may have caused some faulty results. In the future, if essential oils are used as a medication, another question will arise; how will the essential oil be distributed to the right place? Essential oils might have the best effect in its volatile phase, but that’s not possible inside the human body. Methods like capsules and nasal treatment have already been tried, with different outcomes (Chen et.al., 2015, 1580). With this all said, essential oils are clearly antimicrobial and worth doing research about. Hopefully it will bring us natural treatments, with lower toxicity and fewer side effects than ever before. 47 7 References Andrews, J.M., 2001. Determination of minimum inhibitory concentrations. Journal of Antimicrobial Chemotherapy, 48, p.5-16. Atlas, R.M. 2010. Handbook of Microbiological Media Fourth Edition. Boca Ranton: CRC Press. Bakkali, F., Averbeck, S., Averbeck, D., Idaomar, M. 2008. Biological effects of essential oils – A review. Food and Chemical Toxiology, 46, 446-475 Bassolé, I.H., Juliani, H.R. 2012. Essential Oils in Combination and Their Antimicrobial Properties. Molecules, 17, 3989-4006. Berman, J. 2012. Candida albicans. Current Biology, 22 (16), p. R620-R622. Bordi, C & Bentzmann, S., 2011. Hacking into bacterial biofilms: a new therapeutic challenge. Annals of Intensive Care, 1(19), 1-8. Brunke, S., Hube, B., 2013. Two unlike cousins: Candida albicans and C. glabrata infection strategies. Cellular Microbiology, 15(5), 701-708. American Journal of Cancer Research, 5(5), 1580-1593. Chen, T.C., Fonseca, C. O D., Schöntal, A.H. 2015. Preclinical development and clinical use of perillyl alcohol for chemopreventation and cancer therapy. Dalleau, S., Cateau, E., Bergès, T., Berjeaud, J-M., Imbert, C. 2008. In vitro activity of terpenes against Candida biofilms. International Journal of Antimicrobial Agents, 31, 572576. Fidel, P.L., Vazquez, J.A., Sobel, J.D., 1999, Candida glabrata: Review of Epidemiology, Pathogenesis, and Clinical Disease with Comparison to C. albicans. Clinical Microbiology Reviews, 12(1), 80-96 Flores, F.C., Beck, R.C.R., da Silva, C de B., 2015. Essential Oils for Treatment for Onychomycosis: A Mini-Review. Mycopathologia, published online 19 October 2015. Foster. T. 2004. The Staphylococcus aureus “superbug”. The Journal of Clinical Investigation, 114(12), 1693-1696. 48 Gibbons, S. 2003. Anti-staphylococcal plant natural products. Natural Product Reports, 21, 263-277. Gillum, A.M., Tsay, E.Y., Kirsch, D.R. 1948. Isolation of the Candida albicans gene for orotidine-5’-phostphate decarboxylase by complementation of S. cerevisiae ura3 and E.coli pyrF mutations. Molecular and general genetics, 198(2), 179-182. Granger, B. L. 2012. Insight into the Antiadhesive Effect of Yeast Wall Protein 1 of Candida albicans. Eukaryotic cell. 11(6), 795-805. Gutiérrez M.M., Werdin-González, J.O., Stefanazzi, N., Bras, C., Ferrero, A.A. 2015. The potential application of plant essential oils to control Pediculus humanus capitis (Anoplura: Pediculidae) Parasitology Research, published online 14 October 2015. Hare, J. Sabouraud. Agar for Fungal Growth Protocols [Online] http://www.microbelibrary.org/component/resource/laboratory-test/3156-sabouraud-agarfor-fungal-growth-protocols [Accessed 23.09.2015] Hili, P., Evans, C.S., Veness, R.G. 1997. Antimicrobial action of essential oils: the effect of dimethylsulphoxide on the activity of cinnamon oil. Letters in Applied Microbiology, 24, 269-275. Hsieh, M.H., Yu,C.M. & Yu, V.L. 1993. Synergy Assessed by Checkerboard. A critical analysis. Diagnostic Microbiology and Infectious Disease, 16, 343-349. Huovinen, P, Meri, S., Peltola, H., Vaara, M., Vaheri, A., Valtonen, V (eds.) 2013. Mikrobiologia ja infektiosairaudet, Kirja I. Helsinki: Duodecim. Kalemba, B., Kunicka, A. 2003. Antibacterial and Antifungal Properties of Essential Oils. Current Medicinal Chemistry, 10, 813-829. LaFleur, M.D., 2011. Candida albicans Biofilms, Heterogenicity and Antifungal Drug Tolerance. The Open Mycology Journal, 5, 21-28. Lahlou, M. 2004. Methods to Study the Phytochemistry and Bioactivity of Essential Oils. Phytotherapy Research, 18, 435-448. Langeveld, W.T, Veldhuizen, E.J.A, Burt, S.A., 2014. Synergy between essential oil components and antibiotics: a review. Critical Reviews in Microbiology, 40(1), 76-94. 49 Lawler, D.M., 2005. Spectrophotometry: Turbidimetry and Nephelometry. Encyclopedia of Analytical Science. Mayer, F. L., Wilson, D., Hube, B., 2013. Candida albicans pathogenicity mechanisms. Virulence, 4(2), 119-128. Plastics Europe. Polypropylene (PP) [Online] http://www.plasticseurope.org/what-isplastic/types-of-plastics-11148/polyolefins/polypropylene.aspx [Accessed 19.09.2015] Plastics Eruope. Polystyrene (PS) [Online] http://www.plasticseurope.org/what-isplastic/types-of-plastics-11148/polystyrene.aspx [Accessed 19.09.2015] Raut, J.S., Karuppayil, S.M. 2014. A status review on the medicinal properties of essential oils. Industrial Crops and Products. 62, 250-264. Rajeshkumar, R., Sundararaman, M., 2012. Emergence of Candida spp. and exploration of natural bioactive molecules for anticandidal therapy – status quo. Mycoses, 55, e60-e73. Sigma-Aldrich. Introduction to Yeast Media [Online] http://www.sigmaaldrich.com/technical-documents/articles/biology/Introduction-yeastmedia.html [Accessed 23.09.2015] Saad, N.Y., Muller, C.D, Lobstein, A., 2013. Major bioactivities and mechanism of action of essential oils and their components. Flavour and Fragrance Journal, 28, 269-279 The European Collection of Cell Cultures (ECACC), Sigma-Aldrich. ECACC Handbook – Fundamental Techniques for ECACC Cell Lines. [Online] http://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/SigmaAldrich/Instructions/1/ecacc_handbook.pdf [Accessed 20.09.2015] ThermoFisher Scientific, Safety Data Sheet. 2015. [Online] https://tools.thermofisher.com/content/sfs/msds/2012/D12345_MTR-EULT_BE.pdf [Accessed 11.11.2015] Toloza, A., Zygaldo, J., Biurrun, F., Rotman, A., Picollo, M. 2010. Bioactivity of Argentinian essential oils against permethrin-resistant head lice, Pediculus humanus capitis. Journal of Insect Science, 10(185), 1-8. V&P Scientific, INC. The Concept Is Simple. [Online] http://www.vpscientific.com/index.html [Accessed 10.10.2015] 50 Wadhwani, T., Desai, K., Patel, D., Lawani, D., Bahaley, P., Joshi, P., Kothari, V. 2008. Effect of various solvents on bacterial growth in context of determining MIC of various antimicrobials. The Internet Journal of Microbiology, 7(1), 1-6. World Health Organization (WHO). 2015. Antimicrobial resistance. [Online] http://www.who.int/mediacentre/factsheets/fs194/en/ [Accessed 07.10.2015] Appendix 1 Appendices Essential oil components Reference: https://pubchem.ncbi.nlm.nih.gov/search/search.cgi Chemical structure Name Thymol Molecular Formula C10H14O Molecular weight Can be found in Component Group 150.21756 g/mol Thymus Vulgaris- Terpene – Phenol Thyme oil Carvacrol C10H14O 150.21756 g/mol Origanum vulgare – Oregano oil Terpene - Phenol Appendix 1 Chemical structure Name Linalool Molecular Formula C10H18O Molecular weight Can be found in Component Group 154.24935 g/mol Ocimum basilicum - Terpene – Alcohol Basil oil Terpinen-4-ol C10H18O 154.24932 g/mol Melaleuca alternifolia - Terpene – Alcohol Tea tree oil 1,8-cineole C10H18O2 170.24872 g/mol Eucalyptus globulus Eucalyptus oil Terpenoid - Ether Appendix 1 Chemical structure Name Citronellol Molecular Formula C10H20O Molecular weight Can be found in Component Group 156.2652 g/mol Cymbopogon citratus - Terpenoid - Alcohol Lemongrass oil Menthol C10H20O 156.2652 g/mol Mentha x piperita - Terpenoid – Alcohol Peppermint oil Geraniol C10H18O 132.15922 g/mol Rosa damascene Rose oil Terpenoid – Alcohol Appendix 1 Chemical structure Name Molecular Formula Cinnamaldehyde C9H8O Molecular weight Can be found in Component Group 132.15922 g/mol Cinnamomun verum - Aromatic - Aldehyde Cinnamon oil Eugenol C10H12O2 164.20108 g/mol Syzygium aromaticum Clove oil Aromatic – Alcohol Appendix 2 Medium recipes Yeast Extract Peptone Dextrose (YPD) 1 litre o Yeast Extract, granulated, Merck o Bacteriological Peptone, OXOID For Agar: Difco Agar, granulated, BD ˃Add double distilled water until 950 ml ˃Autoclave ˃Add 40% glucose 10 g 20 g 20g Sabouraud (Sab) o Sabouraud 2% Glucose Agar, Sigma-Andrich o Follow the instructions on the bottle Mueller-Hinton II o Mueller Hinton Agar 2, Sigma-Andrich o Follow the instructions on the bottle Rosewell Park Institute Medium (RPMI) 1 litre o RPMI-1640 Medium R6504-1L, Sigma-Andrich 10,4 g o MOPS (C7H15NO4S), Sigma-Andrich 34.53 g ˃Adjust pH to 7 with 4M NaOH ˃Filter sterilize with Thermo Scientific Filtration Product Appendix 3 Abbrevations EO Essential Oil EOC Essential Oil Components EO/C Essential Oil or Essential Oil Component DMSO Dimethyl sulphoxide YPD Yeast Extract Peptone Dextrose MH2 Mueller-Hinton II RPMI Rosewell Park Institute Medium