Multiple SS Adiabatic CSTR
advertisement

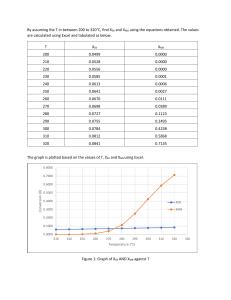
Problem 2: Variation of a problem that we have seen before. This is example problem 8.4 in “Elements of CRE” by H.S. Fogler . We have changed the Cp values and rate constant so that the multiple steady state solution can be illustrated. a. Propylene oxide reacts with water to form propylene glycol. This is done in a CSTR. Propylene oxide is dissolved in methanol (inert material). Water is supplied in excess, and mixed just before entering the CSTR. The water has a bit of sulfuric acid (0.1 wt%) which is a catalyst. However, the properties of the water can be used in the calculations directly, without accounting for the presence of sulfuric acid. There is a small ‘heat of mixing’, but we can account for this and say that the feed is effectively at a slightly higher temperature. The reaction is proposed to be conducted under adiabatic conditions. b. Data: FA-in = 20 kmol/h, FB-in = 200 kmol/h, FM-in = 33 kmol/h, CpA = 120 J/mol/K, CpB = 60 J/mol/K, CpM = 60 J/mol/K, CpC = 100 J/mol/K, ∆HRxn at 20 °C = -85000 J/mol of A reacted. Feed temperature = 27 °C (after −9000 accounting for heat of mixing), k = 17 ×1011 e T h -1 where T is in K. c. The volumetric flow rates of methanol and propylene oxide were 1.5 kL/h each and that of water is 6.5 kL/h. Qin = 9.5 kL/h. The CSTR volume is 1.25 kL. Solution: The volume of the reactor is 1.25 m3, determine the conversion. Here, we know V, but we do not know T or x. The basic equations are V xMB = and Q k (1 − xMB ) FA-in × xEB × -∆HRxn at T = FA-in Cp-A (T-300) + FB-in Cp-B (T-300)+ FC-in Cp-C (T-300) = 17700000 (T-297) The first equation can be written as 1.25 = τ = 0.1316 h −1 = 9.5 x 11 17 × 10 e MB −9000 T which can be re-arranged as (1 − xMB ) −9000 xMB kτ 2.2368 × 1011 e T = = −9000 1 + kτ 1 + 2.2368 ×1011 e T The second equation can be written as x = 0.0104 × (T-300) In the energy balance equation, the relationship between x and T are linear. In the mass balance (design) equation, it is highly nonlinear. If we solve them together, we will get the value of T and x. Another way is to choose values of T (e.g. from 300 to 400) and then plot the value of x given by mass balance equation (xMB) and by the energy balance equation (xEB). When they intersect, we will note that value as the solution for both T and x. Or, we can tabulate xMB and xEB and find where they intersect. T (K) 302.5 340 392.25 XMB 0.0261 0.4165 0.9604 XEB 0.0261 0.4165 0.9605 Thus all the three solutions are possible. If we draw ‘heat generated and ‘heat removed’, then we can easily show that the intermediate point is unstable while the high and low temp points are stable. Right now, crudely, we can think of the ordinate of MB equation as heat produced due to conversion and that of EB equation as heat removed due to fluid flowing out. At the intermediate level a slight increase in temperature will produce more conversion (since XMB is more than the XEB) which in turn will increase the temperature and it will end up at the high conversion. At the low end, an increase in temperature will cause a slight increase in conversion (MB) but more of it will be removed by the outgoing fluid (EB), so the temperature will decrease and come back to original steady state value. Similar argument holds for high temperature steady state also. We can write heat generated as G (T ) = FA−in x ( −delH ) and heat removed as R (T ) = ∑ ( Fi −in C p −i ) (T − Tin ) i