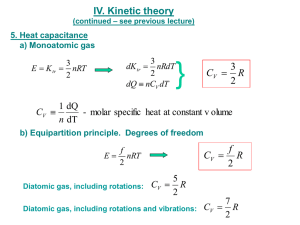
ΔU=QW Recall that ΔU=nCV(Tb-Ta)
advertisement

Ideal gas law: nRT=PV Isobaric process W=PΔV y Q 1st law of thermodynamics: ΔU=Q‐W Recall that ΔU=nCV(Tb‐Ta)=n(R/(γ‐1)) (Tb‐Ta)=1/(γ‐1)(PaVa‐PbVb) in the second step we have used CV=R/(γ‐1) and in the third step the ideal gas law γ=1.4 for diatomic gas γ=1.667 for monoatomic gas. If we are given the adiabatic process then we can find γ=‐ln(Pb/Pa)/ln(Vb/Va) nCV(Tb‐Ta)= 1/(γ‐1)(PaVa‐PbVb) nCV(Tb‐TTa))= 1/(γ‐1)(PaVa‐PbVb) nCV(Tb‐Ta)= 1/(γ‐1)(PaVa‐PbVb) 0 since T does not change =ΔU 0 0 since V does not change =‐ΔU ΔU PVγ=const. t =P(Va‐Vb) Q=ΔU+W =nRTln(Vb/Va) Q=W Since we are given the adiabatic process we first compute the γ for this gas: γ=‐ ln(Pb/Pa)/ln(Vb/Va) =‐ln(5)/ln(1/3)=1.465 So we have a mostly diatomic gas with a little bit of monoatomic gas. Wtot=Wab+Wbc+Wcd+Wda=Wab+0+Wcd+0= =‐(γ‐1)‐1(PbVb‐PaVa)‐(γ‐1)‐1(PdVd‐PcVc)= = ‐(γ‐1)‐1(PdVd‐PcVc+PbVb‐PaVa)= =‐(γ‐1)‐1(PcVc(Vc/Vd)γ‐1‐PcVc+PbVb‐PbVb(Vb/Va)γ‐1) = ‐(γ‐1)‐1(PcVc‐PbVb)((Vc/Vd)γ‐1‐1)= = (γ‐1)‐1(Pc‐Pb)Vb(1‐(Vb/Va)γ‐1) 3(1‐(1/3) 0 465) =(0.465) ( )‐11(10‐5)×100kPa×0.333E‐4m ( ) ( ( / )0.465 =14.3 J Not very realistic (Ta is close to 1 Kelvin) but let’s go with these numbers as an example) Qin=(γ‐1)‐1(PcVc‐PbVb) =(γ‐1)‐1(Pc‐Pb)Vb=35.8 J e=Wtot/Qin=1‐(Vb/Va)γ‐1=1‐(1/3)0.465=0.40=40%