} IV. Kinetic theory R C
advertisement
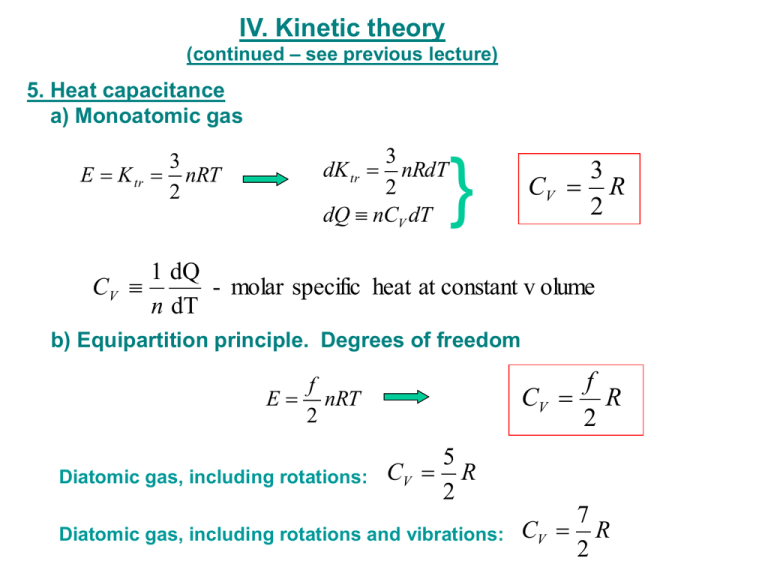
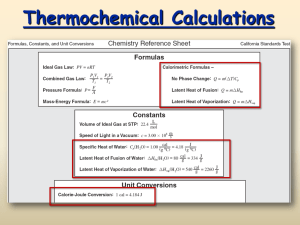
IV. Kinetic theory (continued – see previous lecture) 5. Heat capacitance a) Monoatomic gas 3 E K tr nRT 2 3 nRdT 2 dQ nCV dT dK tr } 3 CV R 2 1 dQ CV - molar specific heat at constant v olume n dT b) Equipartition principle. Degrees of freedom f CV R 2 f E nRT 2 Diatomic gas, including rotations: 5 CV R 2 Diatomic gas, including rotations and vibrations: 7 CV R 2 Molar Specific Heats of Gases at Constant Volume CV At Room Temperature Monatomic He Ar CV (J/mol·K) 12.47 12.47 Diatomic H2 N2 O2 20.42 20.76 21.10 Polyatomic CO2 SO2 28.46 31.39 3R/2 = 12.47 J/mol·K 5R/2 = 20.79 J/mol·K 7R/2 = 29.10 J/mol·K Question How many rotational degrees of freedom does the water molecule have? 1. 1 2. 2 3. 3 4. 4 c) A hint of quantum theory b) Heat capacitance of solids E 3nRT CV 3R 5. Distribution of molecular speeds (Maxwell distribution) 3 dN Nf (v)dv m 2 2 mv2 / 2 k BT v e f (v) 4 2k BT Molar Specific Heats of Elemental Solids at Constant Volume due to Lattice Vibrations The rule of Dulong and Petit only holds at high temperatures. At low T, quantum mechanical effects reduce CV. V. The first low of Thermodynamics (conservation of energy) ΔU = ΔQ - ΔW dU = dQ - dW 1. •Macro- a micro- parameters •Equation of state m for monatomic ideal gas: PV RT •Thermodynamic equilibrium 2. Internal energy (kinetic plus potential energy of particles) dU = dQ + dWext dWext = -dW dW = P dV dU = dQ - dW = T dS - P dV dQ = T dS 3. Work dW Fdx PAdx PdV dW PdV •Work is path dependent •Heat is path dependent •Internal energy is path independent V2 W P(V )dV V1 P P 1 2 1 ΔW = P(V2 - V1)>0 V1 V2 ΔW V P 2 V1 V2 P 2 1 V1 3 2 ΔW = P(V2 - V1)<0 V2 V ΔW 4 1 V V1 V2 V ΔW12 = 0 ΔW23 > 0 ΔW34 > 0 ΔW42 < 0 Example1: A quantity of air is taken from state a to state b along a path that is a straight line in the PV-diagram, where Va=0.070 m3, Vb=0.110 m3, Pa=1.00*105 Pa, Pb=1.40*105 Pa. Assume that the gas is ideal. a) What is the work, W, done by the gas in this process? W = ½ (Pa + Pb )(Vb - Va ) P b W = ½ (1.00 + 1.40)* 105 Pa*(0.1100 - 0.0700 ) m3 a W = 4.8* 103 J V Va Vb b) What happen with temperature and internal energy of this gas? For ideal gas: PaVa PbVb Ta Tb K ~T Tb PbVb 1.40 105 Pa 0.1100m3 2.2 5 3 Ta PaVa 1.00 10 Pa 0.0700m K b Tb 2 .2 K a Ta Example2: This P-V diagram represents a system consisting of a fixed amount of ideal gas that undergoes three different processes in going from state A to state B. Rank work, heat transfer, change in internal, kinetic, and potential energy for each process. P State A ΔW1 < ΔW2 < ΔW3 2 ΔU1 = ΔU2 = ΔU3 = UB - UA ΔT1 = ΔT2 = ΔT3 = TB - TA ΔU = ΔQ - ΔW ΔQ1 <ΔQ2 <Δ Q3 For ideal gas: PE=0 and Δ PE=0 T~ K=U ΔK1 = ΔK2 = ΔK3 = KB - KA 3 I State B V Example3: A system consisting of a quantity of ideal gas is in equilibrium state A. It is slowly heated and as it expands, its pressure varies. It ends up in equilibrium state B. Now suppose that the same quantity of ideal gas again starts in state A, but undergoes a different thermodynamic process (i.e., follows a different path on a P-V diagram), only to end up again in the same state B as before. Consider the net work done by the system and the net heat absorbed by the system during these two different processes. Which of these statements is true? 1. The work done may be different in the two processes, but the heat absorbed must be the same. 2. The work done must be the same in the two processes, but the heat absorbed may be different. 3. The work done may be different in the two processes, and the heat absorbed may be different in the two processes. 4. Both the work done and the heat absorbed must be the same in the two processes, but are not equal to zero. 5. Both the work done and the heat absorbed by the system must be equal to zero in both processes. Note: See example 2 Question 1 One cm3 of liquid water at 100 ˚C is boiled off at 1 atm pressure and is converted to 1670 cm3 of steam at 100 ˚C. The “system” is the water. The work done by the system when it is converted to steam is __ L·atm. (1 L = 1000 cm3) 1. 0.17 2. 1.7 3. 17 4. 1700 Question 2: The work done by the system in going from point 1 to point 2 is ___ L·atm. 1. 2. 3. 4. 1 2 3 3