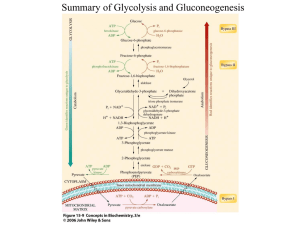
Process for manufacture of milk sugar
advertisement

April 13, 1948. D. D. PEEBLr-:s Er AL PROCESS FOR MANUFACTUÈE 2,439,612 MILK SUGAR Filed Se‘pt. 28, 1942 2 Sheets-Sheet 1 April 1;-, _1948. o. D. PEEBLAES er AL 2,439,612 PROCESS- FOR MANUFACTURE O'F MILK SUGAR Filed Sept. 28, 41942 ` 2 Sheets-Sheet 2 @m ArroeA/ÈY "Patented ~Apr. 13, 1948 " 2,439,612 UNrrEosTA'rEs Hiram'N ortica N q. David D. Peebles, Hillsborough, _and Thomas V. ` Marquis, Santa Cruz, Calif., assignors to West-` ern Conilensing` Company, San Francisco, l Calif., a corporation of California ‘ Application september 2s, 1942, sei-1eme. 459,9zo 8 Claims. (Cl. 12V-31) 1 ` , other insoluble contaminants during crystalliza« This invention relates generally to processes for the manufacture of milk sugar or lactose from liquid Whey. , ` tion. process of the above character having a novel Conventional processes for the manufacture of procedure for effectively separating out lactose lactose from whey are carried out as` follows: A portion of the protein of the whey is coagulated by `addition of lime and application of heat, or by the addition of other coagulants, after‘which the coagulated solids together withprecipitated crystals after the crystallizing operation, and ‘ which minimizes the percentage of contaminants ` removed with the crystals, and also minimizes resolution of lactose. calcium phosphate and other insoluble solids are 10 ‘ removed by decantation, ñltration or centrifug ing. The clarified eliluent is then concentrated by evaporation, and lactose is `crystallized from the concentrate. Lactose is then removed from the ` mother liquor by` centrifuging and further puri ,. Another object of the invention is to provide a ‘ l l ` ` Another object of the invention is to make pos sible the preparation of a relatively pure `lac tose by crystallization from a iacteal` concentrate to which no chemicals like mineral acid have been added. c ‘ ñed by washing. Processes of thischaracterdo A further` object offj the invention is to provide a process making `possible the manufacture of not produce a lactose as pureas desired or as can milk sugar from raw whey. while at thesame time ‘ 15 affording a valuable food product from the >re-. be obtained by the prcsent‘invention, and nutri tive constituents of the whey solids, other than the recovered lactose„are seriously impaired or destroyed. „ . r ` Some lactose has also been manufactured by ` ‘crystallizing from a concentrate of a lacteal ma .maining ingredients. zo Referring to the drawing: , - Figure 1 is a flow sheet illustrating one pro cedure which can be followed in carrying 'out the present invention: and , Figure 2 is adiagrammatic view showing h terial like skim milk, but Without previous re ‘movval‘ of proteins. In such a process, however, 25 draulic classifying apparatus suitable for use in separating out lactose crystals. the proteins' of the lacteal fluid are purposely held Flgures`3, 4 and 5 are curves showing‘the eiîect` " in solution or a‘state of colloidal dispersion and of our special heat treatment. remain so during'the re-crystallization of the lac- ~ In carrying out the present process we evapo tose and its subsequent separation. The process has not proven satisfactory (see Fundamentals of 30 rate whey `to produce a concentrate which is Dairy Science; Rogers, 2nd edition, page 129), ` supersaturatedwith respect to its lactose content. the difficulties encountered being attributed to contamination of the crystals with protein. Ac By means of special heat treatment the concen- t of relatively pure lactose by crystallization from sequent _evaporating step I2. The temperature trate is caused to have certain properties-which we have found-to be conducive to the production cording to our observations such a process is im practical because the crystals formed lack such 35 of relatively large and fast settling lactose crys tals in the/subsequent crystallizing operation. A ` uniformity and size as is required for eiîective hy 'part of the lactose is permitted to crystallize out draulic separation, centrifuging, and washing. In `another process which has been `usedto` ‘ of the concentrate, and this mixture is then sub- ` mitted vto hydraulic classification for removal some extent, the coagulable protein is not re of the lactose crystals. This last step is pref moved prior to crystallization, but is enzymically erably aided by diluting the concentrate with a degraded to molecularly‘smaller `and less readily cool `diluen-t like water. `Finally the removedA lac coagulable `substances in order that crystalliza tose can be centrifuged and washed. tion and subsequentoperations may take place in The procedure shown in Figure 1 is as folli ws: a fluid relatively ‘free of insoluble contaminants. 45 l'A suitable raw liquid material, such ,as einer Such a process is relatively expensive to carry out cheese or casein whey, >or a mixture ofthe two, commercially, it is critical with respect to pH vcon is preheated at l0 and is then momentarily he ated trol, and it requires addition of a chemical like to-an elevated temperature at ll. The heating hydrochloric acid which is later neutralized. ` at I I‘can be conveniently carried out by direct in It is an object of the present invention to pro 50 troduction of live steam into the preheated ma víde a process making possible the manufacture , terial„as this material is being pumped to the sub to which the material is heated is preferably in excess of 212° F., and a suitable range is from 220 agulated proteins. and without any treatment to prevent the presence of coagulated protein or 55 to 260° F. After being abruptly heated to such e, concentrate, without previous removal of co 9,439,612 an elevated temperature by direct contact with dures only- one 4" piece of equipment is used for the -the steam, the material immediately proceeds to the evaporating step l2 where the evaporation is preferably carried out under vacuum, in suitable vacuum evaporating equipment. As the material passes `to the ñrst evaporating stage, it immedi flash heating. Following production of the concentrate, which is characterized by the absence of lactose crys tals, and which contains a particular type of ately- flashes to a lower temperature below 212° F., and such relatively low temperatures are main tained until evaporation has been carried out to, a suitable degree. The heating at l i is for a short interval, and will not discolor or otherwise det- ` ter in a finely divided state, the concentrate is subjected to the crystallizing operation l5. This can be carried out in suitable crystallizing tanks, coagulated protein as well as other insoluble mat rimentally aiîect the whey soli . It is desirable to carry out evaporation until the concentrate containsfrom, say, 50 to 55% ` solids. At such concentrations there is a tend ency for small crystals of lactose to form in the 15 evaporator. We have found that their presence detrlmentally affects the process, presumably be cause their small particle size prevents properly where the material is permitted to remain for a. i period of time sufficient to permit the develop- ` ment‘of lactose crystals. During crystallizing, gentle agitation is desirable, and it is also desir able, but not necessary, to subject the material to gradual cooling. For example, in a typical in stance the concentrate may be received in the ycrystallizing tanks at a temperature of about 110' F., and during crystallization it may be cooled to a temperature of the order of 68° F. controlled crystall formation during the crystal In order to reduce the crystallizing period, and 20 lization step and hinders subsequent separation, in order to facilitate production of relatively centrifuging and washing.` To eliminate such large, fast settling material, it is preferable to small crystals, we pass the concentrate through a. s‘eed fresh concentrate -With material which has heating step I3, like step il, and where the con been treated in the crystallizing tanks. Thus'at centrate is momentarily heated to an elevated temperature such as from 220 to 260° F. by direct 25 the end of the crystallizing operation, one `can remove only a part of the batch of material (such as 25 to 50% of the total mass undergoing crystal heating, the concentrate can rapidly passl through i contact with steam. Immediately following such one or more final stages of evaporation at I4 to cool it to a temperature of the order of 110° F., and to complete evaporation. Steps Il and 'i3 can be carried out by the use of relattively simple equipment. For example, one can -malle use of a small cylindrical cham ber about 14 inches long,.and about 3 inches in ` dlameten-’with a stream of whey entering one end and leaving the'other. Live steam at a pres sure above atmospheric is introduced tangentially into the chamber near the point of introduction of the whey. The flow rate through the cham ber should be such as to afford only momentary or flash heating to the high temperature, as for example a time period within the chamber of. from 2 to 5 seconds. The discharge of this cham so lization) , leaving the remainder to intermix with and seed a succeeding batch of concentrate. , Another procedure which can be used for the crystalllzing operation is as follows: An initial batch of concentrate is crystallizedover a suit->> able period such as 24 hours. ~ Without removing ' any of the initial batch, it is mixed with the next batch of concentrate, and the combined material is further crystallized for a like period. `A third‘ batch of concentratev is then added and crystal lization continued.` as with they second batch. Further additions of concentrate can be mad dependinggupon The material the removed tankage from capacity. the crystallizing ` operation is diluted at I6, preferably with rela. tively cool water. For example, assuming that the material being withdrawn from the crystallizing ‘ ber is led lby piping directly to the expansion 45 tanks is at a temperature of the order of 68° F., chamber of the’vacuum evaporator. the water used for dilution is preferably relatively , The preheating step .l0 is not essential but is colder, `as for example a temperature of 60° F., , desirable in order- to avoid too great a dilution or lower. The extent of the dilution may vary, although in a typical instance from 25 to’50 gal ation Il. Preheating is preferably carried out-to 50 lons of waterv can be used to dilute 100 gallons a temperature of the order of, say, 180 to 190° F. of concentrate, depending upon vthe nature of the by use of suitable heating apparatus such as one concentrate. In place of plain waterfother aque by steam condensate in the heat treatment oper- \ using heated tubes through which the liquid is rapidly circulated. ` ous materials can be used, such as whey.` Dilu- , tion in this fashion serves the useful purpose of The type of vacuum evaporating equipment 55 reducing the viscosity of the material, aswell as utilized can vary in practice, although I prefer reducing its density, thus putting the material to utilize equipment of the type disclosed in in better condition for subsequent separation of Peebles et al. Patents Nos. 2,090,985 and 2,168,362. the lactose. Dilution may not be important Such equipment is characterized by rapid flow of under certain ideal conditions, that is with rela the material through the evaporating effects, and 60 tively low viscosity'and exceptionally large crys by the fact that prolonged-heat treatment is tals. However, such ideal conditions can be ob avoide'd. I . ' > tained only infrare instances. » The-separating operation i1 upon the diluted In place of the procedure described above, a Asingle unit of evaporating equipment can be em 65 material should remove the lactose crystals from contaminants and mother liquor without seri ployed with, say, two or more evaporatin'g effects. After the maior part of the evaporation has been f ous re-solution. It will be noted that because of dilution, the liquid is not saturated with respect completed, the concentrate can be passedl back to its dissolved lactose content, and therefore re through the flash heating operation I I. and then repassed through the evaporating equipment for 70 solution of the crystallized lactose can occur. 'I_‘he rate of solution of lactose as well as its solu the operation Il. Also it is feasible to continually bility decreases with decrease in temperature, and recirculate a batch `ofvwhey through and in series therefore use of cold `diluting water lowers the with the flash heating equipment and evaporat temperature of the material and tends to mini ing unit, until the degree of concentration desired has been attained. In both of the latter proce 75 mize re-solution in the separating operation. Various types of separating methods can be ` ‘ 5 for removal of the lactose. although hydrau turn is -caused to a substantial degree by the pres~ ' ence of partially coagulated protein particles, lic `eiassiilcation is preferred. One desirable form of apparatus for carrying out hydraulic> classifi _ ` which have considerable water of hydration and > cation will be presently described. . which increase the viscosity of the concentrate 'I'he lactose crystalsremovedby hydraulic sep 5 becauseïof their jell-like character. When‘whey aration carry a minimum of proteins and other is concentrated by ordinary vacuum evaporation contaminants, and they can be directly subjected to from 50% to 55% solids, only about 50% to 52% to centrifuging at I8. During centrifuging. `the of the protein is coagulated and the protein par lactose is subjected to washing withfresh water i ticles are caused to have a relatively large curd for further puriiication. The lactose 4as i‘lnaliy l0 volume. Our special heat treatment, by momen removed from the basket of the centrifuge con? tary heating to a, high temperature, serves to ir tains ífromlabout 3 to 10% moisture and can be reversibly coagulate substantially all ofthe pro subjected to drying at I9 to produce the ilnal tein which is readily susceptible to coagulation dried product. ‘ ` y . by heat treatment, namely to the extent of about ^ The eii'iuent from the separating operation I'I 15 60% to 62% of the total protein content. y contains valuable food ingredients. including wa By irreversible coagulation we have reference to such denaturing of the coagulated protein that ter soluble >'vitamins like riboñavin and other members of the vitamin IB complex, and is uti the protein particles do not subsequently imbibe lized to form a saleable by-product. Thus the ` overflow from the separation operation I1 is i water -or hydrate to enlarge their relative curd o volume. During such denaturing there is a sub stantial shrinkage in the particle size of the pro . shown being subjected to drying at 20 to pro duce a final dried product. Such a material is 'useful' as an ingredient in stock or poultry feed. tein, and this shrinkage is accompanied by a loss of water of hydration. Y Figure 2 diagrammatically shows a desirable The curves shown by Figures 3, 4 and 5 serve apparatus for carrying out the diluting and sep 25 to illustrate the effect ,of the special heat treat arating steps I5 and Il. This equipment includes ment. In Figure-3 curve I represents the extent a classifying cone 2l having an inlet pipe 22, of coagulation of protein as concentration pro an overilow launder 23, and an outlet pipe 24. ceeds, using a single vacuum evaporation unit in series With a high temperature ilash heater Pipe 26 connects with pipe 22 for introducing cold- water, and the discharge end of pipe 22 is 30 as previously explained. Curve 2 represents the , surrounded by the collar 2l. The bottom of the extent of .proteincoag'ulation where the whey is cone is provided with a discharge opening nor simply pre-heated _to about 180° F. and then con mally closed by the hinged trap door 29. . centrated by vacuum evaporation at treatment In operating the apparatus -ofFigure 2 and to . temperatures of the order from 125 to 130° F. carry out our preferred process, concentrate en 35 Note that, at about 27 Bé., corresponding to labout 52% solids, the percentage of coagulation shown tering pipe 22 is diluted with" cold water or whey, and as the diluted material enters the cone, lac by curve I is about 62%, while it isonly about 52% for curve 2. According to our_observation tose crystals settle out and collect as a bed in the lower part of the cone. From time `to time it is impossible to coagulate more than about 65% door 29 is opened to withdraw lactose from the 40 of the whey nitrogen as protein, by heat treat bottom of the bed, or if desired the cone may ment. The uncoagulated protein remains in sol be completely filled with lactose crystals, the re uble form. Curves 3 and 4 in Figure 4 show the sidual ei'iluent containing contaminants drained relativecurd volume for the lcoagulatedi protein off, and the entire batch of crystals droppedinto in the concentrates corresponding to curves I a suitable tank. The material is then taken from 45 and 2. Note that at about 27 Bé. the curd volume this tank for centrifuging and washing. The overflow carries off substantially all of the re »of the coagulated protein (curve 3) obtained by use of the momentary high temperature treat ment is substantially less than the curd volume maining solids, including the finely divided co agulated protein. With apparatus of this char of protein obtained by ordinary evaporation acter, re-solution of the lactose is maintained at 50 (curve I). This means that for a given concen tration the whey made by the present process a minimum. particularly because as the lactose settles to the lower bed of crystals. it is immedi has a substantially lower viscosity than concen ately covered and protected against re-solution by subsequently settling solids, and because the quiescent liquid in that part of the cone-occu trate made by ordinary evaporatiomby virtue of the smaller curd volume' of the coagulated 55 protein. condition of saturation with respect to lactose in solution. ~ , ‘ Figure 5 shows the enect of the heat treatment upon the water imbibing properties of the coagu pied by the collected bed of crystals assumes a i lated protein. Curve `5 of this ñgure shows how f A particular feature of the above process is the coagulated protein of whey prepared by our the speciall conditioning of the concentrate used 60 process does not imbibe water to any appreciable ' for the crystallizing operation, to make possible extent upon prolonged holding, while curvel 6 relatively large crystals `of lactose, having aV high settling rate and capable of being handled with shows that the coagulated protein corresponding to curve 4 does imbibe water to a substantial de gree upon prolonged holding. The effect» of this ` trate containing 50% to 55% solids were prepared 65 would be to increase the 'viscosity of the concen out difficulty in a centrifuge. If a whey concen by ordinary vacuum'evaporation. with or without preheating .as previously described, and if one 'then attempted to crystallize out lactose from a trate during the prolonged holding period which - occurs inthe crystallizing operation, while on the contrary with'the irreversible coagulated pro concentrate obtained in this manner, the lactose tein obtained by our process,` the viscosity im crystals would lack uniformity as to size> and a 70 parted by the protein remains constant while the substantial part of the crystals wouldV be'so small that they could not be readily separated out, cen trifuged,and washed. We have found that this is due to a substantial degree to the relatively high viscosity of such a concentrate, which in 75 lactose is being crystallized. thus promoting the production of large-size and uniform lactose crystals as previously described. A low viscosity for the concentrate is also de sirable in that it aids evaporation to the concen 2,439,612 8 tration desired, particularly in that an increasey mass after such seeding, and then separating out» lactose crystals _from the mass of material. :ln viscosity tends to make handling in a vacuum evaporator more diilicult and the evaporating cycle less eillcient.' l ‘ 2. In aprocess for the manufacture of lactose, l the steps of forming a lacteal concentrate super saturated with respect to its lactose content and In a typical instance the whey `introduced into the process had about 6.05% solids, and the solids analyzed substantially as follows: Per cent ` Acid (as lactic) ____ __' _________________ __ Lactose (monohydrate) containing - coagulated milk protein, causing a substantial amount o! the lactose content to crystallize out of solution, diluting‘the concen 5.6 trate with water which is relatively cool com pared to the concentrate being diluted, the amount oi diluting water being suil‘lcient to eiïect ___ _____________ __ 67.0 `Protein ________________________________ __ 14.3 ______ _ 10.0 Undetermined ______________ __ ________ __ Ash ` 3.1 ì a substantial reduction in viscosity and the tem perature of the diluting water being sufilclently At the end of thecrystallizlng operation, from ' ' llow to minimize resolution oi lactose upon dilu tion, and immediately thereafter removing lac tose crystalsI from the diluted material by hy a 52% solids concentrate. the material contained about 19.0% of crystallized lactose. Oi the total lactose content of the original whey, about 35% draulic classification.l yevaporation to form a supersaturated solution oi " lactose, permitting asubstantial amount of the overilow contained about 76.6% oi’ the total solids . - lactose content to crystallize out from the solu tion, adding water to- the bulk of the material ' The lactose after centrifuging and drying analyzed as follows; containing the crystals to an amount sufficient ìto effect- a substantial reduction in viscosity, the water being relatively cool compared to the con Per cent Moisture ____ _________________ ___.’ ____ __ 0.03 Protein Ash ’ _____ 0.22 Lactose _______________________________ -_ 99.17 Acid „ 0.49 '_ , _ _ ` centrate being diluted to minimize resolution of lactose in the diluted mass, and then removing lactose crystals from the diluted material by hy 0.09 draulic classification. The dry> feed material made by drying the from lacteal material containing milk protein and lactose, subjecting' the material to evapora 7-3 tion and heat treatment to form a concentrate supersaturated with respect to its lactose con tent and completely free from lactose crystals, _ 18.7 the major part of the protein concentrate being Per cent Acid ~ ` __ 57.5 - Protein ' 4. In a process for the manufacture oi lactose overñow from the classifier analyzed as follows: Lactose ì from whey, the steps of concentrating whey by trifuging. The feed product made by drying the in the original whey. ' Í3. In a process for the manufacture of lactose Was removed from the lower end of the classifier, and about 34% remained after washing and cen lrreversibly coagulated, seeding a mass of the concentrate with lactose crystals formed in a 40 previous crystalllzing operation of the process, It will be apparent that the products' obtained permitting lactose crystals to form in the seeded will vary in analysis from time to time, depend mass,.diluting the mass of material with water in Ash 13.0 Undetermined _________________________ __ 3.5 ing upon various factors such as the character an amount suiilicient` to effect a substantial re of the raw whey employed, the preciseprocedure duction in viscosity, said water being cool com employed in carrying out concentration and 45 pared to the temperature of the mass being di crystallization, and the extent, of re-solution per luted to minimize resolution of lactose in the di mitted in separating the lactose. The figures luted mass, and then separating out lactose cited above by Way of example demonstrate the crystals from the diluted material. high degree of purity made possible by the 5. In a process for the manufacture of lactose process. 50 - from lacteal material containing ì milk ‘protein In the foregoing reference has been made to and lactose, subjecting the material to vacuum use of ordinary liquid whey such as is obtained evaporation to form a concentrate supersatu in the manufacture of cheese or casein. The rated with respect to its lactose content, ñash` process is also applicable to other lactose con heating the concentrate at about the completion taining -lacteal iiuids, such as a material prepared 55 of evaporation to a temperature and for aiperiod ` by mixing dry powdered whey with water. Such a reconstituted whey can be‘ treated as previously described, with modiiication oi the evaporating operation in accordance with th'e solids content` of the reconstituted material. We claim: . l. In a process-for the manufacture of lactose from a liquid lacteal material containing milkl protein and lactose, subjecting the material to evaporation and heat treatment to form a con centrate which is supersaturated with respect to its lactose content, >which has the major part of its protein content irreversibly coagulated by ‘ of time sufiicient to cause complete resolution >of lactose crystals formed during previous vacuum evaporation, cooling the concentrate after such heating without crystallization of. lactose, seed ing a mass of the concentrate with lactose crys tals, permitting lactose crystals to form in the seeded mass, and then separating out lactose crystals from the mass of material. ' 6. In aprocess for the »manufacture of lactoseA from a liquid lacteal material containing milk protein -and lactose, subjecting theA material to vacuum evaporation and heat treatment to form ` ' a concentrate which is supersaturated with re heat and which is free of> lactose crystals, the spect to its lactose content; which has the major heat treatment including directly contacting the 70 part of its protein contentirreversibly coagu-` material with steam. to rapidly v'transfer heat to the same, seeding a mass of said concentrate with lactose crystals formed in a preceding crystalliz lated by heat and which is free of lactose crys tals, the heat treatment including ñash heating „ oi’ the material to‘a temperature in excess of ing operation of the process, permitting lactose about 212° F. for a period ‘oi time suñicient to from the concentrate to crystallize out in the 75 lrreversibly coagulate the majority oi' the-whey 2,439,012 l0 permitting lactose crystals to iorm in the seeded protein but insufilcient to cause material dis coloration oi.' whey solids, seeding a mass of said mass, diluting the mass of material with water concentrate with lactose vcrystals formed in a in an amount sufficient to eiïect a substantial re preceding crystallizing operation of the process, permitting lactose from the concentrate to crys tallize out in the mass after such seeding, and then separating out lactose crystals from the mass of material. 7. Ina process for the manufacture of lactose from whey, the steps of concentrating whey by evaporation to Iorm a concentrate supersatu duction in viscosity, said water being at a tem perature suillciently low relative to the tempera ture of the concentrate to substantially reduce thetemperature of the resulting diluted mass to thereby minimize resolution of lactose crystals. and then eiiectfng hydraulic separation oi lac tose crystals from the diluted material, said last named step being conducted =by supplying the rated with respect to its lactose content. seed diluted mass to the upper portion of a liquid sep ‘ing a mass oi the concentrate with lactose crys arating column. establishing an overilow oi liquid from the upper portion of the column together tals, permitting lactose crystals to icrm in the seeded mass, diluting the mass of material with 16 with unseparated crystals, and permitting lac tose crystals to separate out into a lower quies water in an amount suilicient to efl'ect a substan tial reduction in viscosity, and then subjecting cent portion ci the column. \ the resulting mass to hydraulic separation to ei-\ DAVID D. PEEBLES. . iect separation of lactose crystals, said last THOMAS V. MARQUIS. named step being conducted by continually sup 20 plying the diluted mass to the upper portion of a liquid separating column, continually with drawing an overilow consisting o1 liquid and un . REFERENCES CITED The following` references are oi' record in the . ille`of this patent: UNITED STATES PATENTS column, and permitting the lactose crystals to 25 separate out into the lower quiescent portion oi Name Date Number separated crystals from the upper portion oi the , \ ‘ the column. . 8. In a process for the manufacture of lactose from whey, the steps of concentrating whey by evaporation to form a concentrate supersatu 30 rated with respect to its lactose content, seeding a mass of the concentrate with lactose crystals, 193,436 Witte ..... _e ..... .__ July 24, 1877 648,490 , Graei! ____________ __ May 1, 1900 Bell ____________ __ Sept. 2l, 1926 Leighton _______ __ Mar. 20, 1934 1,600,573 1,952,017 2,116,931 2,181,146 , Leviton __________ -_ May 10,1938 Peebles et a1 ...... -_ Nov. 28, 1939