Reprinted from the Journal of the American Chemical Society, 1989
advertisement

Copyright
Reprinted from the Journal of the American Chemical Society, 1989, IIl, 5852.
@ 1989 by the American Chemical Society and reprinted by permission of the copyright
owner.
The Structure of Self-AssembledMonolayersof Alkylsiloxanes
on Silicon: A Comparisonof Resultsfrom Ellipsometryand
Low-Angle X-ray Reflectivity
Stephen R. Wasserman,t George M. Whitesides,*,t Ian M. Tidswell,l Ben M. Ocko,I
Peter S. Pershan.*'I and John D. Axe$
Contribution from the Department of Chemistry. and the Diuision of Applied Sciencesand
Department of Physics, Haruard Uniuersity, Cambridge, Massachusetts 02138, and the
Department of Physics, Brookhauen National Laboratory, Upton, New York t 1973.
ReceiuedOctober 12. 1988
Abstract: The thicknesses
of Cl0-Cl8 alkylsiloxane
monolayers
on silicon-silicon
dioxidesubstrates
havebeenmeasured
with ellipsometry
and low-angleX-ray reflection.Although,for any givensample,thicknesses
measured
by the two methods
agreeto within experimental
error,ellipsometric
measurements
are systematically
largerbv approximately'
2 A. ttris difference
may resultfrom variationsin the sensitivityof the two techniques
to the structurcof rhe interfacebetweensilicondioxide
and the alkylsiloxane
monolayer.The X-ray reflectivitymeasurements
providecvidcncethat theseorganicmonolayers
do
not build up as islandstructuresand demonstrate
that the approximatearea projectedbl eachalkyl group in the planeof
the monolayeris - 2l *, 3 A2. Preliminarystudiesindicatethat this techniquecan be usedto followthe changesin the siructure
of a monolayerwhichresultfrom chemicaltransformations.
The influenceof damasethat is inducedbv X-rav radiationon
thesemeasurements
is discussed.
This paperdescribesthe useof ellipsometryand low-angleX-ray
reflectivityto characterizemonolayerspreparedby reactionof
alkyltrichlorosilaneswith the surface silanol groups of silicon
bearinga hydratednative oxide. Our primary objectivewas to
compareestimatesof the thicknesses
of thesefilms obtainedby
u s i n g t h e s et w o t c c h n i q u e s . E l l i p s o m e t r yh a s b e e n e m p l o y e d
extensively
for the measurement
of the thicknesses
of thin organic
f i l m s . r - s X - r a y 'r ef l e c t i v i t r i s j u s t b e g i n n i n gt o b e u s e df o r t h i s
p u r p o s e .r6l A g r c c m e n tb e t $ e e ne l l i p s o . i t r r a n d X - r a r r e f l e c t i v i t yw o u l d h e l p t o v a l i d a r eb o t h t e c h n i q u e s .A s e c o n d a r r
o b j e c t i v ew a s t o e x a m i n et h e s t r u c t u r a lo r d e r o f t h e s es e l f - a s sembledalkylsiloxanemonolayers.As part of this work, we have
attemptedto generatemonolayersthat havea variationin electron
densityalong the normal to the substratesurface. The intensity
of the X-rays reflectedfrom such samplesis sensitiveto this type
of changein electrondensity.6'12The determinationof the electron
distributionin films ostensiblyhavingvariationsin electrondensity
along the z axis would provideone direct measureof order in these
systems.
Previousstudieshave attemptedto verify the accuracyof ellipsometryin determiningthe thicknesses
of organicmonolayers.
For Langmuir-Blodgett monolayers,estimatesof thicknessby
ellipsometry,isotopiclabeling,l3'la
and surfacepressuresl5
are in
agreement.Theseexperimentsdepended,however,on comparisons
of completeand partial monolayersand demonstratedonly that
the thicknessof a monolayeras measuredby ellipsometrycorrelateswith the number of moleculesper unit area in that monolayer and their length. We have reacheda similar conclusion
when correlating the ellipsometricthicknessesof monolayers
preparedfrom a homologousseriesof alkyltrichlorosilaneswith
the relative intensitiesof carbon and silicon observedin X-ray
photoelectronspectroscopy(XPS).t6 This conclusionhas also
beenreachedin relatedexperimentsthat utilized monolayersof
alkyl thiols adsorbedon gold films.rT
Against the backgroundof theseearlier studies,we had two
reasonsto conducta comparisonof resultsfrom ellipsometryand
X-ray reflection. First, thesepreviousstudiesdid not directly
measurethe thicknessof the monolayers.Second,they examined
Langmuir-Blodgett, rather than self-assembled,
monolayers.
The self-assembledmonolayersusedin this work were prepared
by placinga silicon-ilicon dioxide (Si/SiO2) substratein a solution
i Departmentof
Chemistry,Harvard University.
I Division of Applied Scilnces and Departmentof Phvsics.Harvard
University.
$Departmentof Physics,BrookhavenNational Laboratory.
(RSiCl3).'8 The Si-Cl bonds
containingan alkyltrichlorosilane
react with silanolgroupsleand adsorbedwater2O
presenton the
surfaceof the silicon dioxideand form a network of Si-O-Si bonds
of undefinedstructure.2l The resultingmonolayersare bound
c o v a l e n t l rt o t h c s u b s t r i t t ea n d a r e s t a b l e . X P S r e v e a l st h a t n o
c h l o r i n cr c n r u i n 'i n t h c n t r n T h e d e n s i t yo f s u r f a c es i l a n o g
l roups
o n t h c n a t i \ c ( ) \ r d cr : o n l r - - I p e r 1 0 1 2 . 2 2 , 2 3T h i s d e n s i t yi s
e p p r o x i n . l r r t cci.\q u etlt r t h e s u r f a c ed e n s i n o f R g r o u p sw i t h i n
t h c n t o n t r l r i _ \(c\ cr c b c l o r )r . T h c r e m a i n i n gS i - C l b o n d so f t h e
R S i C l r g r o u p 5a p p a r c n t l )r e a c tw i t h w a t e r 2 a n d f o r m S i - O - S i
r u n doTr S i - O H n r o r e t i e s .
E l l i p s o m e t r ra n d l o w - a n g l eX - r a y r e f l e c t i o na r e b o t h o p t i c a l
(l) Azzam,R. M. A.; Bashara,N. M. tr//rpsometryand PolarizedLight;
North-Holland PublishingCompany: Amsterdam,1977and the references
c i t e dt h e r e i n .
(2) Gun, J.: lscovici,R.: Sagiv, J. J. Colloid Interface Sci. 1984, I0l,
201-213.
(3) PorterM
. D . : B r r g h tT
. B . ; A l l a r a ,D . L . ; C h i d s e yC, . E . D . J . A m .
C h e m .S o c . 1 9 8 7 ./ r ) 9 . 3 5 5 9 - . 1 5 6 8 .
( 4 ) T r o u g h t o nE. . B . : B a i n .C . D . ; W h i t e s i d e G
s ,. M . ; N u z z o ,R . G . ; A l l a r a ,
D . L . ; P o r t e r ,M . D . L a n g m u i r1 9 8 8 ,4 . 3 6 5 - 3 8 - s .
( 5 ) A l l a r a , D . L . ; N u z z o ,R . G . L a n g m u i r1 9 8 5 ,1 , 4 5 - 5 2 .
( 6 ) P e r s h a nP
, . S . P r o c .N a t l . A c a d . S c i . U . S . A . 1 9 8 7 , 8 4 , 4 6 9 2 - 4 6 9 3 .
(7) Pomerantz,M.; Segmriller,A. Thin Solid Films 1980, 68, 33-45.
(8) Pomerantz,M.; Segmiiller,A.; Netzer,L.;Sagiv,J. Thin Solid Films
1 9 8 5 .1 J 2 . t 5 3 - 1 6 2 .
( 9 ) R i c h a r d s o nR, . M . ; R o s e r ,S . J . I i q . C r y s t . 1 9 8 7 , 2 , 7 9 7 - 8 1 4 .
( 1 0 ) W o l f , S . G . ; L e i s e r o w i t zL, . ; L a h a v , M . ; D e u t s c h ,M . ; K j a e r , K . ;
Als-Nielsen,J. Nature 1987,328,63-66.
(l l) Helm, C. A.; Mohwald,H.; Kjaer,K.; Als-Nielsen,
J. Europhys.Iztt.
1987.4. 697-'t03.
( 1 2 ) P a r r a u ,L . G . P h y s .R e u . 1 9 5 4 , 9 5 , 3 5 9 - 3 6 9 .
( 1 3 ) B a r t e l l ,L . S . ; B e t t s ,J . E . J . P h y s . C h e m . 1 9 6 0 ,6 4 , 1 0 7 5 - 1 0 7 6 .
( 1 4 ) M i l l e r , J . R . ; B e r g e r ,J . E . J . P h y s . C h e m . 1 9 6 6 , 7 0 , 3 0 7 0 - 3 0 7 5 .
( 1 5 ) S m i t h .T . J . O p t .S o c .A m . 1 9 5 8 ,J 8 , 1 0 6 9 - 1 0 7 9 .
( 1 6 ) W a s s e r m a nS,. R . ; T a o ,Y - T . ; W h i t e s i d e sG,. M . L a n g m u i r , I np r e s s .
( 1 7 ) B a i n ,C . D . ; W h i t e s i d e sG, . M . J . P h y s .C h e m . 1 9 8 99, 3 , 1 6 7 O - 1 6 7 3 .
(18) Maoz, R.; Sagiv,J. J. Colloid InterfaceSci. 1984, 100,465-496.
(19) Abel, E. W.; Pollard,F. H.; Uden,P. C.;Nickless,G. Chromatog.
"/.
1966.22.23-28.
( 2 0 ) v a n R o o s m a l e n ,A . J . ; M o l , J . C . J . P h y s . C h e m . 1 9 7 9 , 8 3 ,
2485-2488.
( 2 1 ) K a l l u r y ,K . M . R . ; K r u l l , U . J . ; T h o m p s o nM
, . A n a l . C h e m .1 9 8 8 ,
60. 169-112.
( 2 2 ) Z h u r a v l e vL, . T . L a n g m u i r1 9 8 7 , 3 , 3 1 6 - 3 1 8 .
(23) Madeley,J. D.; Richmond,R. C. Z. Anorg. Allg. Chem. 1972, 389,
92-96.
(24) Water seemsto be necessaryfor the formation of alkylsiloxane
monolayers.When preparedin a dry nitrogenatmosphere,the preparation
of a completemonolayerrequireslongertimesof immersion(-5 h) than when
the processis performedin the ambientlaboratoryenvironment(-30 min).
0 0 0 2 - 7 8l 6
3 l t s ll - 5 8 s 2 s 0 1 . s 0@
/ 01 9 8 9 A m e r i c a n
se
Chemical Society
Sel^-.4.ssembledMonolayers of Alkylsiloxanes on Silicon
. 1 ., 4 n t .C h e m .S o c . , V o l . l l l , N o . 1 5 , 1 9 8 9 5 8 5 3
t c c h n i q u e sb a s e do n t h e r e f l e c t i o no f l i g h t f r o m i n t e r f a c e s .A l t h o u g ht h c s ct w i t t e c h n i q u e as r e d e s c r i b e du s i n gt h e s a m et h e o r e t i c a l t r e a t m e n t - F r e s n e l ' s e q u a t i o n sf o r t h e r c f l e c t i o n o f
l i g h t 2 s - t h e y m e a s u r ed i f f e r e n tp r o p e r t i e so f t h e l i g h t r e f l e c t e d
from an interface. In addition,the wavelengths
of the light used
h e r e i n e l l i p s o m e t r y( t r = 6 3 2 8 A ) a n d X - r a y r e f l e c t i o n( t r = =
l - i - 1 . 7 A ; d i f f e r e db y m o r e t h a n a f a c t o r o f 1 0 3 . T h e t w o
t e c h n i q u e sa r e a l s o s e n s i t i v et o d i f f e r e n t f a c e t so f i n t e r f a c i a l
s tr u c t ur e .
I
l.
I
Results
Preparationof Monolayers. We preparedalkylsiloxanemonolayerson silicon-silicon
dioxide(Si/SiO2)substrates
bv reacrion
* i t h a l k y l t r i c h l o r o s i l a n euss i n gt e c h n i q u e s i m i l a r t o t h o s ed e s c r i b e dp r e v i o u s l y . l 6 ' 1 8 ' 2B6e c a u s et h e m e a s u r e m e not f X - r a l '
reflectionrequireslarge, flat samples,the siliconsubstratesfor
t h e s es t u d i e sw e r e s i g n i f i c a n t l yl a r g e r ( 2 . 5 x 7 . 5 c m ) a n d . i n
g e n e r a lt,h i c k e r( 0 . 1 2 5i n . ) t h a n t h o s eu s e dp r e v i o u s l y . 2 7
Some
sampleswere,however,preparedon thin (0.01,5in.) substrates.2s
W e e x a m i n e dm o n o l a y e r sp r e p a r e df r o m s a t u r a t c da l k v l t r i c h l o r o s i l a n e( C
s l 3 S i ( C H 2 ) , C H nr , = 9 . l l , 1 4 . 1 5 . l 7 ) . f r o m
I 6 -h e p t a d e c e n y l t r i c h l o r o s i l (aHnTe S , C l l s i ( C l - l2) , , C H : C t { . r .
a n d f r o m a f l u o r i n a t c ds i l a n e( C l l S i ( C H r ) t ( C F . ) , C F , ) .
E l l i p s o m e t r y .T h e t h e o r r o f e l l i p s o m c t r rh a s b e en d i s c u s s e d
i n d e t a i lb y o t h e r s . l ' 2 eH e r e u , es u m m a r i z ec e r t a i ni n r p o r t a n t
d c t a i l sa n d a s s u m p t i o nos f t h e m e t h o d .
Ellipsometryanalyzesthe reflectionof ellipticallypolarizedlight
from an interfaceseparatingtwo media with different indicesof
refraction. This ellipticallypolarizedlight can be represented
as
t h c s u m o f t w o c o m p o n e n t so.n e i n t h e p l a n eo f i n c i d e n c eo f t h e
l i g h t ( p p o l a r i z a t i o n )t,h e o t h e r p e r p e n d i c u l atro t h i s p l a n e ( s
polarization),Upon reflection,the amplitudeand phaseof each
of thesecomponents
is altered,resultingin a changein the overall
p o l a r i z a t i o na n d a m p l i t u d eo f t h e l i g h t w a v e . T h e s ec h a n g c si n
a m p l i t u d ea n d p h a s ea r e r e p r e s e n t e b
d y ' t' h e F r c s n c lr c f l c c t i o n
c o e f f i c i c n tfso r t h e p a n d s p o l a r i z a t i o n sr o. a n d r . . F - l l i p s o n r c t r r
m c a s u r e tsh e r a t i o o f t h e s ec o e f f i c i c n t sp.. T h c : t u n d a r dr c l l t i o n s h i pb
s e t w e e np a n d t h e m e a s u r e d
a n a l r z e r( - 1 )a n d F r r l e r i z c r
( P ) a n g l e sa r e s u m m a r i z e d
i n e q l - - 1 l : e T h c a n g l c . , /r c p r e , c n r :
S t= r " f r , = t i t n ' Jc r p ti J t
rlr
I =.'\
tl)
I = 2P * n/ 2
(3)
the ratio of the changesin amplitudefor the s and p polarizations
of light upon reflectionfrom an interface. The angle A is the
differencein the phaseshifts that are experiencedby each polarization upon reflection.
In order to use ellipsometryto determinethe thicknessof a
monolayer supportedon a substrate,one must compare data
obtainedfrom the monolayer-substrate
systemwith thosefrom
t h e u n c o a t e ds u b s t r a t e . 3 0T h i s c o m p a r i s o ni s s t r a i g h t f o r w a r d .
but differencesbetweenthe substratein coatedand uncoatedform
m a y s k e wt h e e l l i p s o m e t r irce s u l t s .C l e a ns i l i c o n - s i l i c odni o x i d e
has a high surfacefree energy'and,therefore,a high affinit;-'for
b o t h w a t e r a n d o r g a n i cc o n t a m i n a n t sO
. r g a n i cm o n o l a y e r tse r minating in methyl and viny'l groups have low interfacial free
( 2 5 ) B o r n , M . ; W o l f , E . P r i n c i p l e so f O p t i c s . 6 t he d . ;P e r g a m o nP r e s s :
O x f o r d , 1 9 8 0 ;C h a p t e r1 .
( 2 6 ) T i l l m a n ,N . ; U l m a n ,A . ; S c h i l d k r a u tJ,. S . ; P e n n e rT. . L . J . A m .
C h e m .S o c . 1 9 8 8 ,11 0 , 6 1 3 6 - 6 1 4 4 .
( 2 7 ) T i d s w e l lI,. M . ; O c k o ,B . M . ; P e r s h a nP, . S . ; A x e ,J . D . : W a s s e r m a n .
S . R . ; W h i t e s i d e sG, . M . P h y s . R e u .B S u b m i t t e df o r p u b l i c a t i o n .
(28) The inherentcuryatureof thesethin substrates
madeit necessar;-,
lor
t h e X - r a y r e f l e c t i v i t ym e a s u r e m e n ttso,c o n t i n u a l l ym o n i t o rt h e d i r e c t i o no f
t h e a x i s n o r m a lt o t h e p l a n eo f t h e m o n o l a y e r .
( 2 9 ) M c C r a c k i n ,F . L . ; P a s s a g l i aE,. ; S t r o m b e r gR
, . R . ; S t e i n b e r gH, . L .
J . R e s .N a t l . B u r . S t a n d . ,S e c t .A 1 9 6 3 ,6 7 , 3 6 3 - 3 - l ' 7 ,
( 3 0 ) T h e r e f r a c t i v ei n d e xo f t h e S i / S i O 2s u b s t r a r e
v a r i e ds l i g h t l yf r o m
s a m p l et o s a m p l e .T h e o b s e r v e d
r a n g eo f r e f r a c t i v ei n d i c e sw a s 3 . 8 4 - 3 . 8 9 .
The thicknesses
of monolayersreportedhere were determinedwith the refractiveindex of the substrateon which that monolayerwas prepared.
F i g u r e l . T u ' o - l a y c rm o d e l u s e df o r e l l i p s o m e t r v .T h e : i l i c o n : u b : l r r i t c
h a s r e f r a c t i v ei n d e r n r , t h e m o n o l a y e rh a s r e f r a c t i , ' 'icn d c - rn . . r n d t h c
a n r b i e n tl i r h a s r e f r a c t i v ei n d e xn s . T h e i n t e r f a c e sb e t * e c n c - r c hl . r r c r
a r e a s s u m e dt o b e p e r f e c t l i ,s h a r p . F o r t h e a l k y l s i l o x a n cn t ( r n ( r l .cr \r \ o n
s i l i c o n n, 2 i s - 3 . 8 . n , i s - - 1 . 4 - 5a,n d n s i s a s s u m e dt o b e L T h c : n c i d c n t
a n g l eo f t h e l a s e rl i g h t , r p n i.s 7 0 " . T h e a n g l e so f r c f r a c t i o n ,. - ' , J 0 o
a n d @ 2= l 5 o . a r c g i v e nb l S n e l l ' sl a w ( r , s i n @ , = n . \ r n , . ' , - l
e n e r g i e sa n d r e s i s tc o n t a m i n a t i o n . l l l f c o n t a m i n a t i o n o t ' r h c b a r e
Si/SiO. substratc w'eresignificant, we u'ould expccr rhur rhe
t h r c k n e s s c so f ' t h c n r o n o l a v e r sa s m e a s u r e db l e l l i p s o m c t r \ u o u l d
b e t o o s m u l l \ \ c h a v e f o u n d t h a t t h e t h i c k n e s s c st r t ' r h c : c n t r l k r l s i l o r a n cl n o n ( ) l a \ e r sc o r r e s p o n dv e r y c l o s e l i t r ) t h t r : c * h i c h
\ \ c c x p c c t | o r ' . tI r u n s - e x t e n d e dc h a i n o r i e n t e d p e r p c n d i c u l . r r t o
n
thc surf'uce
that is, to the largest plausibic thi'iine.:. .^\
t r a n s - ex t c n c l c de h a i n i s i n a g r e e m e n tw i t h i n f r a r e d n r c e \ u r c n r c n t s
o[ chain geonretrr.]: We conclude,on the basisof thesc nr ir lines
of evidence,that contamination does not appcilr to aif-c.cr he
ellipsometric results in these sy,stems.rl
T h e c o n v e n t i o n a li n t e r p r e t a t i o no f t h c el l i p s o n t e r r i cd r r e i : b a : e d
o n a m o d e l c o n s i s t i n go f p a r a l l e l i n t e r f a c c s s c p a r a t i n g r i r r . t h e
a l k - ' , " l s i l o x a nmco n o l u r c r . r r n d t h e s u b s t r a t e ( F i g u r e I t
The
c l ' c c t i r c l r i n t - i n r t e l rt h i ek . L r b : t n r t e h a s a r e f r a c t i r c i n d c r 1 . . r h e
I I ( l n ( ) l . i \ c rh , t ' r r u n r t , r r n .r tc l n r c t i V ei n d e r n , . a n d t h c . r n t b i c n t
. i l l n t r ' p i l g 1 i-rc, r . r i ' l r l r c t i r ci n d c r n , ( r r h i c h i s a s s u n r c dt t r b c l ) .
. ' . l n . 1 1 i r 'cu r f - l i c ct r r i d c I l t r c r . r '-1r 5
\ i n e e t ' l t c . l l l . , , ' r 1 . r . r . 1 1 - .l .1r1r e
. l l i . : t c - t . r . , e ' : l ' , ' . :l 'r ' : i : : . : t . i : , ; t ( r p , . . l : , , i t d c . iI ] l ( r i ' c . t c c u r a t e
r C n r c \ c n l . : l i , r ,: i l t t . . l : u ! : U r c r r l ' t h Cn t o n L ) l J \ C rI.n p r a C t t C C
$C
.
t . r v c u . e d . l 1 r r , r - i . r r ,n
c rt r r l c l a n d h a r e m c a s u r e da s i n g l cc f f c c t i r e
l - c 1 - r t. li \ec t n L l e\ l ( ) r t h c : u b s t r a t e t h a t c o m b i n e sc o n t r i b u t i o n sf r o m
t h c b u l k : r l i e r r n u n d t h c s u r f a c eo x i d e . 1 6 A l t h o u g h \ \ ' e a s s u m e
t h u t t h c n r o r n t c r f ' a c c sm
. o n o l a y ' e r - s u b s t r a t ea n d a i r - m o n o l a r e r .
a r c p e r l ' e c t l ) s m o o t h . t h e o r e t i c a l a n d e x p e r i m e n t a l s t u d i e ss u g g e s t
t h a t , f o r e l l i p s o m e t r y . r o u g h n e s sh a s l i t t l e e f f e c t o n t h e m e a s u r e d
thickness of the monolayer.3T-3e
Ellipsometry can, in principle, determine both the thickness and
the refractive index cif a monolayer. For the very thin (<50 A)
films examined here. it is not, however, possible to determine both
of thesequantities simultancously.{ We must, therefore, assume
a value for one of'thcnr before calculatins the other. We have
( l l ) T h c c ( ) n t a c t . r n g l e so f w a t e r a n d h e x a d e c a n eo n t h e b a r e s u b s t r a t e
\\crc tJH:() (Jo 4rrl) 0o The contact angles for monolayers prepared from
o c t a d e c r l t r r c h l , r r o s i l a nt O
e TS) were df;zo-= ll2o: dfD J q2"'.
( . l l ) l n f ' r a r e d \ p e e t r o s c o p \ u s i n g p o l a r i z e d r a d i a t i o n s u g g e s t st h a t t h e s e
n r o n o l a r c r sa r c o n c n t c d n e a r l y p e r p e n d i c u l a rt o t h e s u r f a c e ( t i l t a n g l e = l 4
* l3o ). Scc rcf'l8 and 26.
( . . -1l ) O u r c r p c r r r n e n l a l p r o t o c o l ( s e e t h e E x p e r i m e n t a l S e c t i o n ) m e a s u r e d
t h e e l l i p s o m c t r r cc ( ) n \ l i l n l so l ' t h e s u b s t r a t ew i t h i n 5 m i n o f r e m o v a l o f t h e
silicon watcr irorr rhc wutcr in which it had been stored. Using the X-ray
r c f l e c t i o n t e c h n i q u c . r r e h r r , , eo b s e r v e dt h e s l o w ( 2 0 - 2 5 A i n Z + - t r ; b u i l d u p
o f a c o n t a r n i n r r t i n gl r r re r ( ) n i r b a r e S i / S i O 2 s u b s t r a t e .
(34) Carim. \ tl . Dovek. M. M.; Quate, C. F.; Sinclair,R.; Vorst, C.
S c i e n c e 1 9 8 7 . - ' - lj . 6 - 1 0 - 6 l :
(35) Carim. ,\ ti . Sinclair,R. Mater. Lett.1987, J,94-98.
(36) A demonstration of the validity of the use of an effective index of
refraction for thc substratc is found in ref l, pp 332-340.
(37) Fenstcrmaker. (- \ : McCrackin, F. L. Surface Sci. 1969, /J, 85-96.
( 3 8 ) S m i t h , T . . \ u r l a c e . S c r .1 9 7 6 , 5 6 , 2 5 2 - 2 7 l .
(39) The substratcs used in this study had a surface roughnesson the order
o f 3 - 4 A . S e e r ei 1 7
( 4 0 ) T h e s i m u i t a n e o u sd e t e r m i n a t i o n o f t h e r e f r a c t i v e i n d e x a n d l e n g t h o f
the monolaver dependson the precision of the ellipsometric measurement. For
thc films studied here all refractive indicesbetween 1.0 and 2.5 are oossible
for the indcx of the monolaver. See ref 29.
5 8 5 4 J . A m . C h e n t .S o c . ,V o l . l l l ,
i Y o . 1 5 .1 9 8 9
ll'as.rermon et al
c h o s e nt o m o d e lt h e m o n o l a y e ra s a t r a n s p a r e nm
t e d i u mw i t h a
refractiveindexof l.45.atotherinvesrigatori
haveuseda refracrivc
indexof 1.50for organicmonolayers.s
Our valueis approximately
t h a t o f p u r e l i q u i d a n d c r y s t a l l i n ep a r a f f i n s( l . 1 2 - 1 . 4 1 \ 4 b2 u t i s
lowerthan that of high-density
( 1.49-1.55).4rWhile
polyethylenes
o u r c h o i c eo f r e f r a c t i v ei n d e x i s s o m e w h a a
t r b i t r a r l ' .t h c X - r a y
r e f l e c t i v i t ym e a s u r e m e n t(ss e eb e l o w )s u g g e st h a t t h e e l e c t r o n
densityin thesemonolayers
is similarto that of bulk paraffins.#+7
F o r t h e m o n o l a . v - ' e rxsa m i n e dh e r e .a n i n c r e a s eo i ( ) . 0 i i n t h c
assumed
v a l u eo f t h e i n d e xo f r e f r a c t i o no f t h e m o n o l a r c rw o u i d
d e c r e a s ei t s c a l c u l a t e dt h i c k n e s sb y - 0 . 8 - 1 . 3 A { i
F o r e l l i p s o m e t r )w e u s e da h e l i u m - n e o nl a s e r( \ = 6 - l l 8 A )
a s t h e l i g h t s o u r c e .O t h e r w a v e l e n g t hw
s i t h i n t h c ' , i s i b l er e g l o n
w o u l d p r o v i d cs i m i l a rr e s u l t s . sT h e m e t h o dh a sa n a c c u r a c \ , o r t
the orderof +l .\.
Low-AngleX-ra1 Reflectivity'.The reflectirtnof X-nrrs from
s u r f a c e sh6a s b c c nu s c dt o c h a r a c t e r i z teh e s t r u c r u r r rpl r , l p c r t i c ,
o f s e v e r asl ) ' s t e m si n
. c l u d i n gl i q u i d 5 + o -asnt d l i q u i d c n s r a i sj.2 - 5 '
W e a n d o t h c r s h a v ea l r e a d l d c s c r i b e dt h e t h e o r r o f t h i s r c c n n i q u e 5 sa n d i t s u s e f o r t h e c h a r a c t c r i z a t i o o
n f t t t e s t r u c l . u r co f
monolayerspreparedfrom alkyltrichlorosilane".s'27
We will onll'
summarizecertain featuresof the method.
L o w - a n g l eX - r a y r e f l e c t i v i t ym e a s u r e st h e i n t e n s i t y ,R . o f
X-rays that are reflectedfrom a surfaceas a functicn of thc angle
0 bctweenthe incomingX-ray beamand the sample. In general,
t h e v a r i a t i o no f t h i s i n t e n s i t i rw i t h d i s g i v e n b v F r e s n e l ' sl a w s .
The intensityalsovariesas a resultof the changein the diffcrencc
i n p h a s eb e t w e e nX - r a r s r e f l c c t e df r o n r t h c e i r - n r o n o l r r r ,r i rn t i
m o n o l a y e r - s u b s t r ai nt e
tcrlacesR
. i : r c l r r r c dt t r t J ; , , . 1 -.i' ' r i i
a v e r a g ed e r i v a t i v eo f t h e e l c c t r o nd c n s i n a l o n gt i r e n r r r n r r r( l- -r
a x i so f t h e s u b s t r a t cb. i c q . 1 . l l e r e l l r T l-e q - < )r : t h c , - i t . r r r ,i-nc
R = R r l' r - '
f - , d 1 , . . / d - - ,c \ p ( r q - : )d : l :
J-,
?. = -ln\-r sinfi
log R
5l
n:9
n = 11
n:17
10
a2
OJ
06
0.8
1.0
q z ( A ' ')
F i g u r e2 . I n t c n r i n . R . i ' l X - n r r s r ef l e c t e df r o m a l k y l s i l o r a n em o n o l a v e r s
o n s i l i c o n . . ' s i l i cdt rrnt , r r d c\ u b s t r a t c sa s a f u n c t i o no l q , . t h e m r l m e n t u m
c h a n g eo l ' t h e p h o l r r nu p o n r e f l e c t i o n .T h e m o n o l a v e r sw e r e p r e p a r e d
f r o m a l k y l t r i c h l o r r r s i l a n cC: l.r s i ( C H z ) " C H : . T h e t o p s p e c r r u mi s f o r
b a r e S i / S i 0 2 . F . r c hs p e c t r u mi s o f f s e tb y l 0 r f r o m t h e o n ea b o v ei t . T h e
s o l i dl i n c i s t h e c a l c u l a t c dF r e s n e rl e f l e c t i v i t y R
, p , f o r a p e r f e c t l ys m o o t h
s i l i c o ns u b s t r a t c .
Monolayer
--l
T
\
(-1)
(5)
( 4 1 ) A t r a n s p a r e n rm e d r u m d o e s n o t a b s o r b l i g h t a n d h a s a r e a l r c f r a c t i v e
i n d er .
( 4 2 ) C R C H a n d b r _ t c tokf C h e n t i s t y a n d P h y s i c s , 5 6 t h e d . ; W e a s t , R . C . ,
E d . : C R C P r e s s : C l e v e l a n d .O H , 1 9 7 5 .
(43) Polymer Handbook; Brandrup, J., Immergut, E. H., Ed.;John Wiley:
N e w Y o r k , 1 9 7 5 :V - l 3 - V - 2 2 .
(44) Ny,burg, S C ; Liith, H. Acta Crystallogr. 1972, B28,2992-2995.
( 4 5 ) C r i s s m a n , J . M . ; P a s s a g l i a ,E . ; E b y , R . K . ; C o l s o n . J . p . J . A p p t .
Crystallogr. 1970, J, 194-1 95.
(46) The average electron density in these monolayers was -0.30 A-1.
T h i s v a l u e i s s l i g h t l y l e s st h a n t h a t o f c r y s t a l l i n e n - p a r a f f i n s t 0 . : Z A - r ) . t h e
latter value was calculated from the crystal structures of octadecane ((
ixHr*)
a n d e i c o s a n e( C z o H r z ) g i v e n i n r e f 4 4 a n d 4 5 ,
( 4 7 ) w e c a n o b t a i n a n a p p r o x i m a t e ' a i u e i o r t h c r e f r a c r r r ei n d e r , r l ' r h c
monolaye-u
r s i n g t h e e l e c t r o n d e n s i t r o f t h e m o n o l a \ e r . p c r .d e t er m i n c ' d o r t h c
X - r a y r e f l e c t i v i t ym e a s u r e m e n t s .i h i , e l e c t r o nd e n s r r r c a n b c c o n \ c r r c d r ( )
a mass-density,pr. An alternate form of e9 7 (:ee dr:ct-rr.rrrnon thc pro.lected
a r e a o f a l k y l s i l o x a n eg r o u p s ) i s g i v e n b 1 e q i , w ' h e r eI , ' i s t h e r o l u m e o i e a c h
V = . d = .\',/ p,t
hydrocarbon tail in the monolarer. Since the massof each tail. n. is simply
t h e s u m o f t h e m a s s e so i e a c i r a r o m i n t h e t a i l . t h e m a s s d e n s i t y o f i h e
m o n o l a y e r c a n b e c a l c u l a t e d . T h e r e f r a c t i v e i n d e x o f a s u b s t a n c ei s r e l a t e d
to its molar refractivitv. R, massdensity,and molecular weight, }7, by eq ii.
( n 2- l ) / ( n 2 + 2 ) = R p ^ / w
S i ,S i O 2 ' 0 3S i ( C H2 ) " CH 3
(i i )
R is foundby summingthe refractivities
of eachmethyleneand methylgroup
i n t h e h y d r o c a r b o tna i l ( V o g e l ,A . I . . / . C h e m .S o c . 1 i 4 8 , 1 8 3 3 - 1 8 5 5 ) . - T h i s
c a l c u l a t i o ny i e l d sa n a p p r o x i m a t er e f r a c t i v ei n d e xo f 1 . 5 0+ 0 . 0 7 .
(48) The changein thickness
of the monolayeras a functionof its assumed
refractiveindexis a linearfunctionof the lengihof the monolayer:rhe longer
t h e m o n o l a y e rt,h e g r e a t e rt h e c h a n g ei n t h e c a l c u l a t e dt h i c i c n e s s .
( 4 9 ) B r a s l a uA, . ; D e u t s c hM
, . ; P e r s h a nP,. S . ;W e i s sA, . H . ; A l s - N i e l s e n .
J . ; B o h r ,J . P h y s . R e u L
. e t t . 1 9 8 5 ,5 4 , I 1 4 - l 1 7 .
(50) Bosio,L.; Oumezine,M. J. Chem.phys. 1984,80,959-960.
( 5 1 ) S l u i s ,D . ; R i c e ,S . A .
" r . C h e m .P h y s . 1 9 8 3 , 7 9 , 5 6 5 8 - 5 6 7 2 .
( 5 2 ) O c k o ,B . M . ; B r a s l a u A
, . ; P e r s h a np, . S . ; A l s - N i e l s e nJ. . : D e u r s c h .
M . P h y s . R e u .L e t t . 1 9 8 6 ,J Z , 9 4 - 9 7 .
(53) Ocko,B. M.; Pershan,P. S.;Safinya,C. R.; ChianC,L. y. ph.t,s.Reu.
A 1 9 8 7 .. t J . 1 8 6 8 - t8 7 2 .
( 5 4 ) P e r s h a nP, . S . ; B r a s l a uA, . ; W e i s sA, . H . ; A l s - N i e l s e nJ ,. p h v s .R e t t .
A 1 9 8 7 .J 5 . 4 8 0 0 - 4 8 1 3 .
(55) Als-Nielsen,J. Physica 1986,140A, 3l.6-38"t.
(56) pa is the volumedensityof electrons:that is, the numberof electrons
presentin a unit volume.
t
dpu,
dz
a2
I
Z --------+
F i g u r e . 1 .\ 1 , r r l i i .r., \ i 1 , . t h c c l c c t r t r n
d c n s i t l .a n dd p r l fd : . t h e c h a n g e
t n c l e e l r i ) l( rl . n \ t i \ . : , , n gl i r ! n , i r n t i tpl e r p C n d t C Utl O
l r tr h e p l a n eO f t h e
r t t ( ) r t ( ) l l t \ ct rl \. c ( l ! i ) . , r tl l \ z e t h e n r c . r . t r r c d\ - r l r t r c f l e C t i v i t fo' f a l k y , l s i l o r a n en t r ) n ( ) i i r \ cor \n S r S r O . : u b s t r i l t c \ T h c a i r - n t o n o l a v ear n d
m o n o l a v c r - s u b s t r l rrtnc t c r l J e c \J r c r c p r c s c n t c d
r n d , 1 , .d, - - b r G a u s s i a n
l u n c t i o n sA
. , e r p \ : ) 1 2 o , 2 1a n d 1 2 c r p ( ( :
d 11
) )o2)). The parameter
d . t h e s e p a r a t i o nb e t w e e nt h e c e n t e r so f ' t h e s ef u n c t i o n s .r e p r e s e n t st h e
distancebetweenthe air-monolayer and monolayer-substra'Le
interfaces.
'fhis
d i s t a n c ei s t h e t h i c k n e s so f t h e m o n o l a y e r . A t , A z , a 1 ,a n d o 2 z t r t h e h e i g h t sa n d w i d t h s o f t h e G a u s s i a nf u n c t i o n s . T h e p a r a m e t e r s6 p ,
a n d 6 p 2a r e t h e c h a n g e si n r e f r a c t i v ei n d e xa c r o s se a c h i n t e r f a c ea n d a r e
p r o p o r t i o n a lt o . 4 , o 1 a n d A 2 o 2 . T h e e l e c t r o nd e n s i t y d e c r e a s e sf r o m
s u b s t r a t et o m o n o l a y e rt o a i r . T h e i n d e x o f r e f r a c t i o n f o r X - r a y s i s a
l i n e a r f u n c t i o n o f t h e e l e c t r o nd e n s i t v .
momentum experienced by the X-ray photons during the reflection
process,5w
T hile p- is the electron density of the bulk substrate.
Re is the Frcsnel reflectivity, the intensity of X-rays reflected from
a bare substrate whose boundary with a vacuum is sharp and
perfectly smooth. lf thc refractive index of the substrate is known,
the form of RF is determined solell' by the Fresnel reflection
coefficients. This index of refraction is calculated from the critical
angle, d., for total reflection of the X-rays.ss The refractive index
in the X-ray region is a linear function of the electron density.
( 5 7 ) T h e m o m e n t u mp, , o f a p h o t o ni s p = 1 ,1 5 . S i n c el r i s a c o n s t a n t ,
m o m e n t u mc a n b e r e p r e s e n t ebdv t r - 1 .w h i c h h a s u n i t so f A - 1 .
-.1ssembIedM onoIa1'er.s
.\'el_1
o/',1I kyIsiIoxanesitn S i Iit'on
l,lo. 15.1989 5855
t , . r . 5 sT h e c h a n g ei n e l e c t r o nd e n s i t yd p " 1df z i s t h c r c l i r r ca d i r c c l
r l c a s u r eo f d n l d ; .
[ . q u a t i o n4 d c s c r i b e st h c p a t t e r no f i n t e r f e r c n c ct h a t r c s u l t s
1 ' r o ntrh e r c f l c c t i o no l ' X - r a y s f r o m a n a r b i t r a r l ' e l e c t r o nd i s t r i b u t i o n ./ r . i ( : ) . I n t h e c a s eo f t w o s h a r pi n t e r f a c e s e p a r a t e db v
sorncdistance,eq 4 reducesto the familiar intcrfercncccondition
i o r r c f l c c t i o nf r o m p a r a l l c ls u r f a c c s . s e ' 6S
0i n c ct h e m e a s u r e d
interfercnccpatterndependson the actualdistanceseparatingthe
t u o i n t c r { ' a r c ci ns o u r m o n o l a y e rs y ' s t e n trh, i s m e t h o d .u n l i k ec l l i p s o m e t r l ,d. i r e c t l , vm- e a s u r e st h e t h i c k n c s so f t h c r . n o n t , l u
cr.
O u r e x p e r i m e n tu
s t i l i z e dt w o m o n o c h r o n t a t r / . '-trr.1l 1 i ' g r( )r l
\-rars: a rotating
a n o d c( } . = 1 . 5 4A ) r r n d t h , :\ . , ' , ' " ' r ' \ \ n c h r o t r o nl - - i g hSt o u r c e( N S L S . X = l . 7 i A ; n ' e p r c : e n tt h c d r r r . r
o b t a i n e df r o m t h e s et w o s o u r c e sa s a f u n c t i o no f I i - . e e a r r t' ch c
interference
patternis invariantin q-, regardlcss
of'l hc ri.trcicrigi;'
o f r a d i a t i o nu s e d . W e w i l l a l s ou s u a l l vp r e s e not r , r d . , i . L I i i ,
f o r m R / R p . S i n c ef o r a s i n g l e s, h a r pi n t c r f ' a c cR = R r . " R R ,
= I for all qr. Any divergenco
e f p . 1f r o m t h a t e h r r r r l e l c r , / r ! . r
s i n g l e i,d e a li n t e r f z r cies , t h e r e f o r er,e a d i l r a p n i r r c n.i r sr r c , .i,' . r , ) i .
in R/Re from a horizontal ine.
T h e i n t e r p r e t a t i oonf t h e o b s e r v e idn t c r f c r c n c lcr r i t er r r I i g L r i c
2 s h o w st y p i c a ld a t a ) r e q u i r e sf i t t r n gi t t o a s t r u c l u r . inl ' r , r d c . , , '
t h e m o n o l a y etrh a t i n c o r p o r a t ccsh a n g e si n t h c c l c e i r , r nd c n \ r r \
a l o n gt h e s u r f a c en o r m a l( d p . 1 r ' d : 1\ \ ' c h a r c l n . r l r z c do u r d u t . i
u s i n ga t r e a t m e ndt e s c r i b e idn d c t u i lc l s o r h c r c nt;n d \ u n t m i r n z c d
g r a p h i c a l l yi n F i g u r c3 . T h i s t r r o - l a r c rn t t r d c il : t h c : i n r p l c s t
plausiblemodelfor thc dcscription
of thc alkrlsiloxane
nronolarers.
b u t i t i s n o t a n e x a c tr e p r e s e n t a t i oonf t h e m o n o l a v e r - s u b s t r a t e
s\stem. The presence
of the surfaceoxideon the siliconsubstrate
m i g h t s u g g e s tt h e u s e o f a t h r c e - l a y e rm o d c l , T h e e l e c t r o n
dcnsitiesof amorphoussilicasand bulk siliconare, however,ver)'
sirrilar.62To the X-rays.the siliconand silicondioxidethereforc
a p p c a r ,t o a f i r s t a p p r o x i m a t i o na, s a s i n g l em a t e r i a l w i t h n r r
s e p a r a t i n gi n t e r f a c e . 6 l I n t h i s p a p e rw e w i l l u s e t h c t w o - l a r r r
modelto determinethe thicknesses
of the alkylsiloxanc
mon()larcrs.
p a p c r 2rTv cd i s c u s st h e u n c e r t a i n t i crsr s s o c i : r t e
ud
ilit
In a separate
t h i s m o d e la n d d e m o n s t r a theo l vt h e t h i c k n c sos l t h c n r ( ) n ( ) l i l \ e r
d e p e n d s l i g h t l yr l n t h c r n o d c lu s c d .
O u r r n o d eI d e s c r i b c d
: r , - ' d - -i - o rea eh i n t c r f ' . icer r '
f u n c t i r t n -. l er p ( - - l 1 , r lr i h c n r r r t l cCl r r f l t . i i nt '.r cr
t h c t h i c k n c : s . . r lt h c n r \ ) n ( ) l . r \ c -, r/ .t . r c t u l r l , rt | t c d l ' l
t h c e c - n ' . e rr.r i ' 1 i l c : u t r r 'l.- .i1e : l l , , n ! \ ] \. a1 l - , : : . C j: ,t ' . , , l - , ,
l c r l . : r ' c . , :. h e : e : g i - ' '.i l i . r s l ( i . 1u . . , . 1 1 .1 . : 1 , , 1 . . . : f - . u
l t . c . * : J : :. .
' . l n d r : r i T l ' . e ' l . t r i i m e t e r \ r r - p r r ' \ c n tl h c r ' r u S h : . r : c . . : - . 1
i n t r i n s r cu t d t h s o i h l t h i n t e r i a c c s . T h e c h a n g e st n c l c c l r t r nd c n . r t r
a c r o s sc a c h i n t e r f a c e . 6 p l a n d 6 p r . a r c p r o p o r t i o n a l t o . - 1 1 o ,a n d
. . - 1 2 or.e. s p e c t i v ' e l y T
. h e p o s i t i o n so f t h e m i n i m a i n t h e X - r a 1 ' p r o f i l c
a r e d e t e r m i n e d a l m o s t e n t i r e l y b y d . T h e t h i c k n e s so f t h e m o n olayer can therefore be determined to an accuracy of - I A. The,
amplitudes of the minima, as well as the generai shirpeof the
profile of the scattered X-rays. reflect the combined effects ol A,,
. 4 2 ,o t , a n d o 2 . B e c a u s e t h e s e p a r a m e t e r s a r e c o u p l e d . o b t a i n i n g
( 5 8 ) T h e i n d e xo f r e f r a c t i o nt o w a r dX - r a y s ,n , i s g i v e nb 1 e q r r r . T h i s
n = | - ( 2 r ) - t ) , 2 p , 1 r 0 r o = 2 . 8 1 8x l 0 - s A
tiirt
relationis derivedfrom the classicaltheoryof dispersion
for frequencies
much
higherthan the resonance
frequencies
of the electronsin the sample.Seerel
2 5 , C h a p t e r2 .
( 5 9 ) S e et h e a p p e n d i xi n t h e s u p p l e m e n t a rm
y aterial.
( 6 0 ) H a l l i d a y ,D . : R e s n i c kR
, . P f t y s i c s3. r d e d . ,P a r t 2 J o h nW i l e - v -N: e w
Y o r k , 1 9 7 8 :S e c t i o n4 5 - 5 .
( 6 1 ) D e s c r i p t i o nosf t h i s m o d e la n d m o r e s o p h i s t i c a t em
d o d e l sf o r t h c
s t r u c t u r eo f a l k y l s i l o x a nm
e o n o l a y e ras r e f o u n d i n r e f 2 7.
( 6 2 ) F r o m t h e m a s sd e n s i t i eos f s i l i c o na n d q u a r t zw e c a l c u l a t ee l e c t r o n
d e n s i t i eosf 0 . 7 0a n d 0 , 8 0A - 3 ,r e s p e c t i v e l yS. e v e r am
l i n e r a lf o r m so f s i l i c r .
i n c l u d i n go p a l a n d c r i s t o b a l i t eh,a v ed e n s i t i e sI 5 % l e s st h a n t h a t o l q u a r t z
S e er e f 4 2 .
( 6 3 ) I n c l u s i o no f a t h i r d l a y e r f o r t h e s i l i c o nd i o x i d eo n t h e s u b s t r a t e
improvesthe overall agreementbetweenthe observedreflectivityand that
predictedby the model,but it doesnot havea significanteffecton the measuredthicknessol the monolayer. The presenceof this layer in the modc'l
a l t e r st h e c a l c u l a t e dt h i c k n e s sb y n o m o r e t h a n 0 . 5 A . S e e r e f 2 ' i
(6a) We refer to 01dnd o2as the roughnesses
of the two interi'aces
rn our
m o d e l . T h e y a c t u a l l yp r o v i d ea m e a s u r eo f t h e d i s t a n c e so v e r w h i c h t h e
refractiveindex changesfrom n6 (or n1) to n1 (or n2).
- cHz
o
f,
a 6
o
. i l c r
iI ' o
lri
.l
A-
.
B"
.i
-T----1-l--_]--l
iilrr
10 0 c
0
290
B i n d i n q E n e r g y( e V )
I ilirrr' {.
l'\
".
' : i
'
:
i , l ( ) n ( ) i : r \ cpr r c p a r e d
iromCl35i(CH2)ltCHl
: ' , . . 1 , t " , . , r r ! , i r . l t r . c t lb r C \ p ( ) s u r et 0 X - f a y s f r O m a s ; - n - ' r - " . : , \ ' . , ' l r ' . - r : - , \ i \ r ' ! r i . ,r i c l t t r n d h i g h r c s o l u t i o ns p e c t r ao f t h e
(
\
.'i:.' "
. , . l i r t . , l g g r ) l l h c s ; t t r t n l tch a t h a d n o t b e e n
!"'
" ' l t l r l t : ', t t i l r t . r C U i r , n* l l s l / l l l O= 1 1 2 o .
I
\
r ' \ i r ,\ : 1 1
l,ti..
ir.
-
\\crcindistingUfshable
t \ , 1 ' : . i r : . , . . - :I : 1 . t . . : : . . 1
- l i . : l t . . 1 1: 1 ,I ' - r c ne c t p o s c d t o a n \ X - r a y r a -
.:
\ : 1 j, ! \ . : ' r , , C ' . r . : . . : i . . , , 1i i r e \ . r n r p i ct h a t h a d b e e n e x p o s e dt o t h e g r e a t e s t
' : 1 11 r - r l\ ' : . r ' , .
I:tc c{}nlilelangle in this region was gf;:o = 82". The
h i g h - r c s r r l L r r i , r(n l \ s p c c t r u m e x h i b i t s a t a i l t o h i g h e r b i n d i n g e n e r g ) ' ,
r n c i r e a t r n gt h e p r c s e n c c o i o r i d i z e d c a r b o n s p e c i e s .
r e l i a b l cv a l u e sf ' o r t h c m i s t e c h n i c a l l yc o m p l c r . r '
betueenthe bare
X-ray reflectivitydoesnot utilizecomparisons
sf the
s u b s t r a t ea n d t h c c o a l e ds a m p l et o m e a s u r et h e t h i c k n e s o
in susceptibilitv
to contanrinati()n
between
monolaver.DiI'lc'rcnccs
r o u l d t h e r c f o r eh a r e n o e f f e c t
t h c s u b s t r r i l cr r r r ttl h c m o n o l a y e w
lengthof the monolayer.Adsorptionof impurities
on thc ntca\rrrc(l
( ) l tt h c l l r ( ) r r ( ) l l r r$crrt u l d .h o w e v e rc.a u s ea n i n c r e a sien i t s a p p a r e n t
i h r r ' k n r ' . . J ) u r n ! rt i r e. c r c i ' r rhl o u r sr e q u i r e df o r t h e a c c u r t t u l a t i o n
, r 1l l e \ - ' , . , i . ' 1 .\:\ t ' i r , r r t ' r r b s e r tvhccdb u i l d u po f a c o n t a m i n a n t
'SiOr
s u r f a c c . t t\ \ ' c h e r en o t d e l l I t ' l , , i i ] r , i r ! : r r ' iu r r r ' i r rS i
l c f l . ' i l . r . . r l '. , ' " . r ' l , , , i , ' , , ,r r h c n i t m o n o l a \ e r i s p r c s e n t .
\ - r u r I ) r r n r a g t ' .\ \ : ' e r i l r p s o m e t r l i s a n o n d c s t r u c t i v et e c h ' - , l . r n rt rn, t o n o l a Y
v eertrO
o nn
o s l n c h r o t r o nr a d i a t i O
t - . , i . .! \ ' a . \ . ' r , . t . r . , : . : - i r : r t r l r r r nr r l t h c s a m p l e . T h e e x p e r i m e n t s
-i'i.,-', : .-".::.'tedundcrair ratherthan in vacuum
. \\ i lr,untlthrrt.upttn removal from the
':,,'
:- :l!..
i ' \, r ;; ,' .. 1 gr1r-n r l f l c t h \ ' l - t e r m i n a t e d
\"-''-,'
i l r ' l i op l1t t. ;',1l/ffl l: :(() =) I l ] oo t t lt 0p jI t O
- -- .: ,\ .ta: a ^
_
l : . r . . r : : i d - , r i . : . : . r : r g i c. t n n c i r r c rdr n l r o n t h e
,c' .c- n
n ?t rr ri l. l p
nl , t ) r 'l ir ) r l, r l l h c . . 1n t p l c
th.rl t.. lhc.rrc.rthet had been
crposcdto thc !rcatc:t tlur tlt'.\-rar'. Thc cdgc oi this sample,
w h i c hh a d h a d l i t t l co r n o ex D o s u rteo X - r a r s .c r h i b i t e du n c h a n s e d
w e t t a b i l i t i ( H r r : t=t I l l o ) . F - l l i p s o m . t rfyr i t . . t t . r d i r e c r ni n r
s i g n i f i c a ndt i f f e r e n c cb e t w ' e etnh e d a m a g e da n d p r i s t i n er e g i o n \ .
F i g u r e4 r e p r e s e n tX
s P S s p e c t r ao f t h e C l s p e a k sf r o m t h e
centerand cdge regionsof a monolayerpreparedfrom dodecylt r i c h l o r o s i l a n(eC l r s i ( C H z ) " C H : ) . T h e d a m a g e da r e a ,w h i c h
h a d / / H , o- 8 : o . s h o w sa t a i l i n g t o h i g h e rb i n d i n ge n e r g yt h a t i s
not prescntrn thc areasunexposedto the radiation. We suspect
t h a t t h c s cc h r r n g c isn l J t { : oa n d t h e X P S s p e c t r ar e f l e c to x i d a t i o n
t r l t h e n r ( ) n ( ) l ict r\ t o p o l a r . o x v g e n - c o n t a i n i nfgu n c t i o n a l i t i e s
( . rl c o h t r i s .k c t o n c s .c ur b o x y l i c a c i d s , h y d r o p e r o x i d e sa, n d / o r
trthcrs)6'' \\'c couid ntlt detectthesenew oxygensignalsdirectly
b r \ l ' } S i t g i r r n \ tt h e l a r g e b a c k g r o u n ds i g n a l f r o m t h e o x y g e n
.rtr)lr\in tirc .url.recsiliconoxide. This typeof damageapparently
X - r a y s . S a m p l e st h a t h a d o n l y b e e n
f e ! u r i c : c \ p ( ) s u r ct o r n t e n s e
c\fnsctl to rudirrtionfronra rotatinganodesource,whoseflux was
0 l'i of that of the synchrotron,
exhibitedno change
rrppro\rinirtcli
r f l l / l i - oo r i n \ l ' S s p e c t r a . 6 T
(6-i) Two rarnplcs were analyzed under a helium atmosphere. Similar
darnage was t.rbserv'ed
for these samples as for those analyzed under air. The
c h a r n b e r h o l d r n g t h e s a m p l e sw a s n o t , h o w e v e r ,a i r t i g h t .
(66) I he bindrng energies,relative to CHr, for oxidized carbon atoms are:
C H 2 o l l . + 1 . 5 e V ; C O , + 3 . 0 e V ; C O z H , * 4 . 5 e V . G e l i u s ,U . ; H e d e n . P . F :
H e d m o n . J . ; t - i n d b e r g ,B . J . ; M a n n e , R . ; N o r d b e r g , R . ; N o r d l i n g . C . ; S i c g
bahn.K. Phls. Scr.1970.2.70-80.
5856
J . A n r . C h e n t . . S o t .' L . o l l l l .
.\rt
Ll'asserntonet al
15.19,\9
ComDlele
30
i@
o
E
o
g
uJ
20
.?.
)
Partial Monolayer
./o
j
-
I
I
Subslrate
-0
o
ah
o
Mcnolaver
l)nl:Orm
lslands
10
lr oa.!ra
Il '
l'l pnrt'ai < nr
.9
F
l=,l
Length<dI-
10
20
| --:-=:
30
Thickness(A) by X-rayReflectivity
F i g u r e5 . C o m p a r i s o no f t h c t h i c k n e s s eosf a l k y l s i l o x a nm
e o n o l a \ er : a :
nreasurcd
b r e l l i p s o m e t r ra n d X - r a y r e l l e c t i v i t y .T h e s o l i dc t r c l c s( O )
a r e t h e t h i c k n e s s cosf c o m p l c t cm o n o l a y e r st h
; e o p e nc i r c l e s( O ) a r e t h e
t h i c k n c s s cosl p a r t i a lm o n o l a y e r s T
. h e s o l i dl i n e i s t h a t e x p e c t e di f t h c
t w o t e c h n i q u e sl i e l d t h e s a m et h i c k n e s s .T h e d o t t e d l i n e i s o f f s e tb v 1 . . 1
A a n d i s t h a t e x p e c t e di f o n l y e l l i p s o m e t r yi n c l u d e st h e s i l i c o na r o m o l
t h c a l k y l s i l a n ei n t h e m e a s u r e dt h i c k n e s s( s e et h c t c x t ) .
Although the damage to the monolaver was clearly measurable.
w e d o n o t , f o r t w o r e a s o n s .b e l i e v c t h a t i t h a d a s i g n i f i c a n t e f f e c t
o n t h e v a l u e o f t h e l h i c k n c s : n r e . r s u r e df o r t h c l l r ( ) n r ) l i l \r e I . i . ' 1
s a r n p l e se x a m i n c d o n b t l t h l h e r o t r r t r n gi l n ( x l c . t n t ' l1 i r t . . , , t i
c x h i b i t e ds i m i l a rr e f l e c t i r i t i c -Ss c. c o n di.h c i n l ' , r n r r . r r : ,,'li :' ' : : r r . : - .
i m p o r t a n cien d e t e r r n i n i nt h
c c t h i c k n e r . , ri lh' c n t r , l , . : \ J : ' . r . i : . !
t h e t w o - l a 1 cm
r odel theposition
i r i t h c l ' r: . : l t l r l : . 1 \ : l : : l l - - t u r - - l
i n t h er e f l e c t e d
X - r a r\ - \ \ a \ d c r i rc t j . i l l c r r e i J t i \c l r b r r r f c' \ n ) \ L r r
t o t h e X - r l l rs , 6 n
'fhickness
of {lkrlsilorane \Ionolarerson Silicon. \\ e .rpplied
b o t h \ - r a r r e f l c c t ri rt r ; i n d e l l i p s o m e t rt\o a s e to f a l k r l s i l o r a n e
r r i o n o l i i r e rr.F i s u r c 5 | F o r l - i s a m p l e sa n d s i r c h a i n l e n - q t h s .
t h e a c r c c m c nbt c t \ \ e e nt h e t * r t t e c h n i q u ei s g o o d . T h e m a x i m u m
d e r i a t i o nb e t r r e e nt h e t h i c k n e ses s t i m a t e du s i n gt h e t w o m e t h o d s
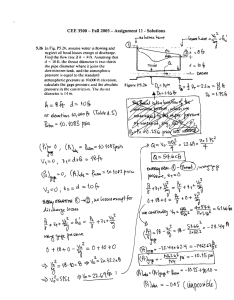
r r - 1L 4 . . t h e a r e r a s ed i f f e r e n c ei s 2 . 2 A ( r m s ) . T h i s a c c u r a c y
t s e q u i r a l e n t o a n e r r o r o f - 1 0 % , i n t h e m e a s u r e m e notf t h e
thickncso
s i a C1i monolayer.
E l l i p s o m e t r \s v s t e m a t i c a l lgy i v e sl a r g e r v a l u e so f t h i c k n e s s .
This differencecould result from the use of too low a value for
the refractiveindexof the monolayer.We would.however,require
n = 1.55in orderto obtainvaluesfor the width of the monolayers
f r o m e l l i p s o m e t r yc o m m e n s u r a t ew i r h t h o s c f r o m t h e X - r a y
m e a s u r e m e n t sW
. h i l e s u c ha h i g h r e f r a c t i v ei n d e xi s f o u n d f o r
crystallinepolyeth,vlene.a3
it seemsunreasonable
for a hydrocarbon
m o n o l a y e trh a t - c o n t a i nm
s e t h rI g r o u p s .
We believethat the discrepancvbetweenthe thicknesses
inferred
f r o m e l l i p s o m e t r iacn d X - r a r m e a s u r e m e n itss, a t l e a s ti n p a r t ,
t h e r e s u l to f a s u b t l ed i f f e r c n c ei n t h e t w o m e t h o d s .T h e e l l i p s o m e t r i ct h i c k n e s s easr e b a s e do n d i f f e r e n c e si n m e a s u r e m e n t s
o f t h e b a r es u b s t r a t e
and the substrate
w i t h a n a t t a c h e da l k y l s i l o x a n em o n o l a r e r T h e r e f r a c t i v ei n d e xo f S i O 2i s 1 . 4 6 6aen d
t h e c o n t r i b u t i o no f a n O . , S i C H 2m o i e t l t o t h e i n d e xo f r e f r a c t i o n
o f t h e m o n o l a r e ri s p r o b a b l rv e r v c l o s et o t h a t o f t h e a l k y l c h a i n
R . T h u s . t h e t h i c k n c s sm e a s u r e db v e l l i n s o m e t r vi n c l u d e st h e
s i l i c o na t o mo f t h e a l k rl s i l o x a nger o u p I n t h e X - r a y e x p e r i m c n r
t h e m e a s u r e dt h i c k n e s sc o r r c s p o n d tso t h e d i s t a n c es e p a r a r i n p
i n t e r f a c e sb e t u ' e e nm c d i a o i d i f i e r e n te l e c t r o nd e n s i t i e s .S i n c c
t h e e l e c t r o nd e n s i t ro f t h e s i l i c o na r o m i n t h e R S i O 3g r o u pt h l r
a t t a c h e st h e m o n o l a \e r t o t h e s u b s t r a t ei s e f f e c t i v e l vi n d i s t i n (67) We were able to causedamage to these monolayerswith a rtrraring
anode by placing a monolarer in rhe direct beam of thc anode for 24 h. The
f l u x o f t h i s b e a m w a s - r , . , 0 t h a t o f t h e m o n o c h r o m a t i z e dr a d i a t i o n f r o m t h e
synchrotron. While the contact angle in the irradiated region decreased(d"H:o
^- 80"), XPS failed to find a significant surface concentration of oxidizcd
carbon atoms.
(68) Sample deterioration will bc important. however, in more detailed
studiesof how monolayer structure changesunder various conditions.
(69) Tait, E. A.
" / . E l e c t r o c h e m .S o c . 1 9 7 8 . 1 1 t . 9 6 8 - 9 7 1 .
F i g u r e 6 . M t l d c i t 1 ' r ' ' i r r ' s l l - r r s i i . l rr c) l l n ! \ r l l l p l c t en l o n o l a y e r s . A c o m p l e t c m o n t t l i l \ i ' r 'i r . : . . . t i r t I i r c . ' , . i r . ] r r 1 !.l r n r n d c x o f r c i r a c t i , r n . n , I n t h e
u n i t ' o r n rm o d e i l t : e i ' . r r i r . i r; r ' , r n o i a r e rh a s a i e n g t h l e s s t h a n r / a n d a n
i n d e x o f r c f ' r a e t r . , I. l1 l n r ( ) \ t n l r r t c l cr q u a l t o n 1 . I n t h e i s l a n d m o d e l t h e
i n c o m p l c t e m ( ) n ( ) i : r \ c rh l r s ; r t h i c k n e s s . d . b u t t h e i n d e x o f r e f r a c t i o n i s
lessthan rr,.
guishablefrorr thlt of thc oxidelayeron the substrate.the silicon
a t o m o l t h e a l k l l s i l a n eg r o u p a p p e a r st o t h e X - r a y s t o b c p a r t
o f t h c s u b s t r a t en. o t o f t h e h v d r o c a r b o nm o n o l a y e r . I n s h o r t .
c l l i p s o n trre! r r r l r s U r ctsh t ' t h i c k n e sosf r r S i t C H , ; , C F l , m o n o l a l ' e r ;
\ - r - , r r r n t t ' j r \ i l i ' fl h ' 1 i , r . l ( ( I l . ) - (
Il
nt(ln()l:lvcr Thts crnlrrnatiOn
. :1..rll:'irr .itltrld hC - 1..1
\
\-rt'. ::f i'tt\ll\
-.:-',.
fhcsc
-'.r-,'i :.
: - - t . : t l , - . r , 1t h c , r l t r c r r c d d i l t ' c r c n c e
' - , , ' ' . ' . e ..' 'r- . . . . : . : . r , - r i r , i r1- .: T l r c r c n r . l i n r n ld: r f f ' e r c n c c
\ : ' : . . :r - - , : , r , . r r :r t l ei , . r ef r , , r.f r n t h c n t r l d e l su s g d
. : l . 1: l , i t r z l l . - f : : l i
-',
: 1 . , l l. el i : u
.:ld
\-f
.tr d.ttlt.
Projected \rca of \lkrlsilorane (,roups in the Plane of the
\ l o n o l a r e r . I h c d r r t r rf ' r ( ) n lro $ - a n g l e X - r a r i c a t t c r i n g p r o r i d e s
a s en r i q u a n t i t a t i rc e s t n l l a t eo f t h e i n - p l a n e a r c a o l ' c r r eh . r i k r l s i l o x a n e g r o r l p i n t h c s e n r o n o l a v e r s . T h e c r i t i c a l a n g l c l ' t ) rt o t a l
r - e f l e c i i o nl - r t l r r ti h c s u b s t r a t c . 0 . . i s r e l a t e d t o t h e e l e c t r o n d c n s r t , r
o f t h e s i l i c o n s u b s t r r t c p . r , ,( c q 6 ) . " T h e o b s e r v e dc r i t r c a l a n g i c .
0.)= tr);t,1rn/r
r,1=2.818 x l0-54
(6)
0 , = 0 . 2 2 5 + 0 . 0 0 7 o l i . r rX - r a v s h a v i n g w a v e l e n g t h X = l . 5 4 A , 7 1
c o r r e s p o n d st o a n c l e c t r o n d e n s i t y o f 0 . 7 2 * 0 . 0 5 A 3 . T h e e x p e c t e d v a l u c f o r s i l i r : o n .( ) 7 0 , 4 3 . i s i n g o o d a g r e e m e n t w i t h t h i s
n u m b e r . T h e f i t t r n g o i l h r p r o f i l e o f s c a t t c r e dX - r a 1 ' st o t h e m o d e l
o f ( d p " 1 i d : ) f ' t l r t h c r " o rc r c d s t t h s t n t t cg t v c s i t n e s l i m a t c c - t tf h e
e I e c t r o n d e n s r l \ ( ) l i i r ( ' : r ] i u r , r l . i \ c r/./ - . . . r - ei a t i v e t t t t h a t t l f t h e
s u b s t r a t e .F t r r t h c r r ' , r I k , t 1 1 1. .i', r 1 , r i . l r cs'tiu' :d i c dh c r c . ' , i ce : i i m a t e .
u s i n ga t h r e e - i a r c rl n ( ) d c . i i t r i l r , . . . , . ,1 , . - t=r 0 - 1r + 0 . ( ) - s . : - r l T h e
a r e a p e r a l k l l s i l t r r r r r i cf r r r : f ' . l . , - . : n t i t c n b c c a i c u l a t e df r o r n t h i s
e s l i m a t c o t ' t i r c c l c r t r - o n, j c ' t . t l r t r l t h c n t o n o l a re r . t h e t h i c k n e s s
o f t h e m o n o l ; t \ r r . , . i .. r n L li i r t n u p r b c rr t f ' c l e c t r o n sA, ' . , i n t h e a l k y l
g r o u p o l e t t c h , r l L rl . r l , r \ , r r r i 1 ( r i c t \ ( c q 7 1 . 7 r O u r c a l c u l a t e dv a l u e
| = .\',/ dp,1
(7)
l i r r I r s - l l + I \ r p c r R S i g r o u p . T a A n a l t e r n a t i v e a n a l y s i s ,b a s e d
-r)r
r
l h c \ r 1 t :\ ) l l h c s i l i c o n a t o m i s t a k e n a s h a l i t h e s u m o f t h e p r o i e c t i o n s
: i r i .\ r t ) r i { . 1A t a n d S i - C ( 1 . 5 1 . \ ; h o n d so n t ( )l h e : a x i s . T h e s e
I ) r i ) t c c l l o n sw e r c c a l c u l a t e df r o m s t a n d a r d b o n d l e n g t h sa s s u m i n ga b o n d a n g l e
, r ' l 1 ) 9{ o
t - l ) T h i s v a l u c f b r t h e c r i t i c a la n g l ei s s l i g h t l r h i g h e rt h a n t h e v a l u ed " =
r ) l l l p r e d i c t e db y t h e e l e c t r o nd e n s i t v t r l - c r r s t a l l i n es i l i c o n ( 0 . 7 0 A - 3 ) . W e
, r t t n b u t e t h i s d i s c r e p a n c vt o c u r v a t u r e i n t h e r a m p l e . S e e r e f 2 7 .
( 7 2 ) T h e e l c c t r o nd e n s i t vo f t h e n r o n o l a y e r 0
, .30 + 0.03 A-1, calculated
u i t h t h e t h e o r e t i c a l c n t i c a l a n q l c f o r s i l i c o n ( r e f 7 l ) , c o r r e s p o n d st o a m a s s
d e n s i t l ' o i 0 8 7 + ( l l 0 g , ' c m t T h t : d e n s i t v i s c a l c u l a t e da s s u m i n gt h a t t h e
n r o n o l a v e r c o n s r s l ss o l e l v c i m e l h . , , l e n eg r o u p s .
( 7 3 ) l ' h c n u m b e r o f e l e c t r o n si n e a c h c o n s t i t u e n t m o l e c u l e o f t h e m o n o iavcr was iound bl adding thc nunrberofelectrons contributed by each atom
i n t h e t a i l o l t h c s i l a n e .s i x f o r e a c h c a r b o n a t o m a n d o n e f o r e a c h h y d r o g e n .
t74) The major sourceof uncertainty in this estimate is the uncertainty
i n t h e e l e c t r r i nd e n s i t r o f t h e m o n o l a v e r .
,'
\t'lr- .l.isenthled Monolat'er.s ol' Alkt,lsiloxanes on Silir.on
2-
,z
Complele (x 100 )
" l , 4 r r t (. ' h e m .. S o r ' .V, o l . I l 1 , N o . 1 5 . 1 9 8 9 5 8 5 7
A
l'
t
.----
\A
I
.R
,oS
0RF
ros#;
-2 -
-4 -
B
2-
/-
Complete ( r 100 I
I
.R
rog
0.2
'.
0R,
-2 .
-4
t\
0.4
0.6
0.8
q, (A-1)
r n r t h c c l c c t r o nd e n s i t yo f t h e a l k y l s i l o x a n e
. , , r r c l l c e t e dX - r a y s , R / R p : ( A ) S i / S i O 2
. : ' r - c dt ' r r r nCr ' l , S i ( C H 2 ) 2 ( C F 2 ) 7 CaFn3d,( C )
: r r ( i l - t " ( I l , \ u n d B a r e o f f s e tb y f a c -
Partial
-
0
0.2
0.4
0.6
0.8
q. (A-1)
Figure7. Comparison
of the X-rav reflectivitv,
R/Re, of partialand
e o n r p l c tner o n o l a y eprrse p a r eidr o mC l 3 S i ( C H 2 ) r C H(3A:) n = l 7 a n d
r B ) a = l l . T h er e f l e c t i v i t ioefst h ec o m p l e tm
e o n o l a y ear sr eo f f s e bt y
r r f a c t o ro i 1 0 0 .
( ) nm o n o l A V e rtsh a t h r i d b e e np r e p a r e df r o m d o d e c y l -a n d o c t a dccrltrichlorosilane,
yieldsan areaof 22.5 + 2.5 4.27 Thcscareas
. i r c s i m i l a r t o t h a t f o u n d f o r c l o s e - p a c k eLda n g m u i r - B l o d g e t r
r n o n o l r l \ ' e rosf l o n g - c h a i na l c o h o l s( 2 0 . 5 - 2 2 A ? f s a n d t o t h r :
c r o s 5 - s e c t i o naar lc a p e r m o l e c u l ew i t h i n c r v s t a l so f l t t n s - c h u r i t
p a r a f f i n s( 2 0 . - 5A 2 ) . 4 a , aOsr h c r s t u d i c sh a v ec o n c l u d c dt h i t r h c , c
s c l f - a s s c n t b lsetdr u c t u r eisl r e t h e m s e l l ' east ( ) r n c i l rI e l ( ) \ c - p i l c k c d
i i r r a n g e m e n t . lO
s u r r c s u l t sa r e c o n s i s t c n\ t\ i t h t h i : r , r r r . r c l t r : r t t n
Structureof Incompleteh,
Formed\Ionolaiers. \\ c u,'L,,ldlik.
t o b e a b l c t ( ) i r \ \ e \ \t h e p r t t c c sbsr r rh i eh . r l k rl t r i eh r r ) - r r' .rr i . - .
, t d s r r rlbr n db r : d 1 , , I . i l i , r r ns u b r t r : r t c \-\ i t . , c. r c t . . r t i t , , i, .r t l
O u reU r r L ' nl C
t r c ,l r l l e eh n r e . t: i r n h l r l t s . t l t rdr:nr .c e
l : \ . t l . 1 lzr r i i t r
: r , ) L i l \ \\.\ j i . : : d c t c - : t t : :cc' : r : . i : lnc , l : u t c . . , : : : l c\ : : t . . : . . : J :
. n c ( r n r l 'l .ec l rl ' r t r n t e|d1 . 1
r l l J i ) m r ) n o l aL\. r \ T h c . t n . i 1 1 .,1r 5; l i i g . g
: i f u C t u r c \n l a \ . i n t u r n . r h c d l r g h t( ) nh o \ \ c o m p l c t cn l o n ( ) i l t \ L ' r \
lirc forntcd.
\ \ ' e g e n e r a t e dp a r t i a l m o n o l a y e r sb v r e m o v i n gt h c s u b s t r a t e s
lrom the solutionscontainingthe alkyltrichlorosilanes
beforethe
monolayershad formedcompletely.We hypothesized
two extreme
possibilities
for the structureof such monolayers(Figure 6). A
completemonolayeris characterizedby a length, d, and a ref r a c t i v ei n d e x ,n , . I n o n e p o s s i b l es t r u c t u r ef o r a n i n c o m p l e t e
monolayer.the alkyl chainswould be uniformly distributedover
t h e s u b s t r a t eb u t w o u l d b e d i s o r d e r e da n d h a v e a l i q u i d l i k e
s t r u c t u r e . I n t h i s " u n i f o r m " c a s e ,t h e m o n o l a y e rw o u l d h a v ea
r e f r a c t i v ei n d e x s i m i l a r t o t h a t o f t h e c o m p l e t em o n o l a v e r b, u t
its thickness
wouldbe less. In the secondstructure,the monolayer
w o u l d c o n s i s to f i s l a n d so f ' a l k l l s i l o x a n eg r o u p s h a v i n g l o c a l
s t r u c t u r es i m i l a r t o t h a t o f t h e c o m p l e t em o n o l a y , . e rI.n t h i s
" i s l a n d "m o d e l ,t h e t h i c k n e s w
s o u l d b e r h e s a m ea s t h a t o f t h e
c o m p l e t em o n o l a y e r ,b u t t h e a v e r a g er e f r a c r i v ei n d e x o f t h e
m o n o l a y e rw o u l d b e l o w e r . W e c a n n o t .u s i n ge l l i p s o m e t r rd, i s t i n g u i s hb e t w e c nt h e s ep o s s i b i l i t i e ss,i n c er v c r n u s ra s s u m et h e
r e f r a c t i v ei n d e x o f t h e m o n o l a y e ri n o i - d c r t o d c t e r m i n e i t s
t h i c k n e s s .X - r a y r e f l e c t i o nc a n , h o w e v e rd. i f f e r e n t i a t cb er r v e e n
thesetwo models. For a structurecontainingisland:.thc ptrsitions
o f t h e m i n i m a i n t h c X - r a y p r o f i l ew o u l d b e t h e s a m ca s t h o s c
o f t h e c o m p l e t em o n o l a y e rs i n c et h e d i s t a n c e sb e t w ' c e n
thc airmonolayerand monolayer-substrate
interfaceswould be the samc.
The intensitiesof theseminima would changebecausethe averase
( 7 5 ) G a i n e sG
, . L . . J r . I n s o l u b l eMonolayers at Liquid-Gas
I n t e r s c i e n c eN: e w Y o r k . 1 9 6 6a n d references cited therein.
I nterface.s.
c l c c t r t l nd c r t . t t , ,' , ri l i . rt t i l t e ; ' j . 1 1 1- g1 ,1r 1 l i . i i n t n\gt r U C t U r ew O U l db e
I o r v c rt h a n t h . r t i i r t h r n t h c e ,) n l n l c l cn t ' r n r ) l uc\ t - F - o rt h e " u n i f o r m "
s t r u c t u r e t h e d r s t l r n e c\ c p i t r i i t l n s l h c r n t c r l i t c c \ \ \ t t u l d b e l e s st h a n
t h a t o f t h c c o n r p i c t cr . n t t n o i a r c r .T h c r c t ' t t r c t. h c l o c a t i o n so f t h e
m i n i m a w ' o u i d d i f - f c r f r t t n t t h o s c o l ' l h c c t r n t p l c t cm o n o l a v e r .
Figurc 7 shows thc intcnsitl of X-ra\\ rcflcctcd from two
monolay'ersprepared from octadecvltrichlorosilanc(Cl35i( C U r l r ? C H r . O T S ) . T h c c o m p l c t c m o n o l a \ c r \ \ i - r \p r c p a r e d b y
i n r n t e r s i n gt h c s i l i c o ns u b s r r a t ei n a s o l u t i t l nc o n t a i n i n gO T S f o r
I h . l t h . r d . r t i r r e k n c \ \ .b \ e l l i p s o r n c t r r o. t - l t ' . \ . T h e s e c o n d
\ J r . ) ) p l cr r . 1 ' p, l , i g 1 ' r'ir t t h c : r r n r c s t ) l u t i o nl i t r - 1 0s . B r e l l i p s o m e t r y
itr lir.k'r, '. , . r \
i r n r ( r r i n t l r lt re 6 0 ' l o f ' t h a t o f t h e c o m p l e t e
\ l r u ! l i t i ' - [ ' . - r . . . ' 1. , i . . t , , 1 sr :h i l ' tl n t h e p o s i t i o no f t h e p r i m a r y
r l : r : r r , i i : , ' r . ' . : ' , i . . - : i '. i n d p l l r t i a l m o n o l a y e r s . T h i s s h i f t
t, irJ.i',.r.,,:
: : J : J : r . t , r i . { i n t h i c k n e s sw. h i c h i s w e l l
'rC,,,:t.::-_
, \ , : . . :: . - . - - , r r- , l t l i e C \ p e f i n f C n t .
I -.-.
, . : ' - . - - ' ' . ' l l . . : : t j . l i l rl i r r l $ 0 n t o n o l l r c r sf o r m e d
:".. ( ,:rr( tl-r (ll,r \\hilethisset
- i - - i - , ' l : . . , 1i , ' : t h c n t t r n o l a r epr rse p a f e d
'
. ' t
,:rl ,. :tl.:rer:
\\ hilc in this latterSVStem
i i l Cl i ' . g , , r t ' : ' u : ! ' - :i \:\i . i ' J . l t . : . efi: U \ c n t c du s f r O r nO b t a i n i n g
r c l r l r b i cr . ,l [ r e. i ' \ l -' . h cc ] c el r ' , n d en \ ; ' . \ , t l 't h c n t t t n o l a l e rt ,h e
s r n r i i a r i t tr n t h c . t r n p l t t u d ctsi l l h c r n r n l n t .\iu g g c s t st h a t t h e
c l e c t r o nd e n s r t ro l ' t h c i n c o n t p l e tnct o n o l a r c\r\ a s s i n t i l a rt o t h a t
o f t h e c o m p l c t cs t r u c t u r c .
W e c o n c l u d ct h a t t h e s t r u c t u r eo f t h e s ep a r t i a l m o n o l a v e r iss
b e s td e s c r i b e db , " t-h c " u n i f o r m " m o d e l ( F i g u r e6 ) . 7 6 T h i s c o n clusiondiifers from that of Sagiv.77
which is basedon an infrared
studyof partial (-60%,) and completemonolayerspreparedfrom
O T S o n a l u m i n u m b y p r o c e d u r e s i m i l a r t o t h o s eu s e d h e r e .
Variation of the ElectronDensity of the Monolayer. The intensityof reflectcdX-ravsat the interference
minima in the X-ray
profile is smallestu,henthe intensitiesof light reflectedfrom the
substrate-monolal'cr
and monolayer-airinterfacesare equal. This
c o n d i t i o ni s n r c t w h e n t h e e l e c t r o nd e n s i t yo f t h e m o n o l a y e ri s
a p p r o x i n r a t c lhr a l f u a l ' b e t w e e nt h a t o f t h e s i l i c o ns u b s t r a t ea n d
a i r . l f ' t h c e l c e t r o nd e n s i t ro f t h e o r g a n i cl a y e ri s t o o c l o s et o t h a t
o l ' t h e s u b s t r r r t{c) r ( ) f a i r . t h e i n c o m i n gX - r a y ss e eo n l y o n e i n t er f a c e: t l r u t h r l i n g r t s i g n i f i c a n tc h a n g ei n e l e c t r o nd e n s i t y .
W ' c h l r c r l c r . n o n s t r a t et hdi s e f f e c t b y c o m p a r i n gt h e X - r a y
profilcs l'..rrnr () Ill()n()lalersformed from alkyltrichlorosilanes
( 7 6 ) \ \ e ( l ( ) I r r ) lg c n e r a l t z et h i s r c s u l t t o o t h e r m o n o l a y e r s y s t e m s . T h e
a l k r l t r r c h l o r ( ) s r i ; l n cl r\r r 1 1,1m o n o l a y e rt h r o u g h t h e c r e a t i o no f c o v a l e n ts i l i c o n o x \ g c n b r , n d sb c t r r e e nr h e s i l a n ea n d t h e s u b s t r a t e . O n c e b o u n d t o t h e
\ u b s t r i i t c t h e n t r r l e c u l c sc a n n o t m o v e a l o n g t h e s u r f a c e . F o r s y s t e m ss u c h a s
thiols on gold. *here thc monolayer is held on the substrate by weaker intcriictions. it rnar bc possiblelbr the monolayer constituents to diffuse laterally
r r c r o s st h e s u r l ' a c e
t 7 7 ) C o h e n .S
N a a m a n ,R . ; S a g i v ,J . J . P h y s . C h e m . 1 9 8 6 , 9 0 ,
r05.1 1056
Wasserman et al.
5 8 5 8 J . A m . C h e m . S o c . .V o l . l l I , N o . 1 5 . 1 9 8 9
o
-
)
j
r \
Y
|
|
r o gR
+
.
o ^
N ^ + :
! r
-
n
Qilz
"
-'@
;
|
m
l,r
|
3
t{|| |,
rA
li tr.ir'
o
l
0r
o
o
lm'llllr
,rl
- "CHBTCHTBT
I
I
I
-2 -
o
l
- "qtlBrC,H28r"
L,l
,t l
t
J
-4 -
q
a
co2tt
t-
/\,
I
- co2il
R
.
r09 p-
'l
0-
Il
"F
looo
-2 -
286
5fo
242
e i n o i n qe n e ? g yl e v y
-4 0
0.2
0.4
0.6
0.8
q. (A'1)
Figure9. Changein the intensityof reflected
X-raysthat resultsfrom
monolaycrs
chemical
transformations
of vinyl-terminated
alkylsiloxane
p r e p a r e fdr o m C l 3 5 i ( C H 2 ) 1 . C H : C H(rH T S ) : ( A ) a d d i t i o no f e l e m e n t abl r o m i n e( 2 % .v ' . t 'i.n C H l C l l ) t o f o r mC H B r C H , B ro r r c l a t e d
b r o m i n a t es dt r u c t u r ea sn d( B ) o r i d a t i obnr K \ 1 n O .t,0 5 n r \ l ) \ e l O . ,
( 1 9 . 5m M ) / K 2 C O(.1 . 8m M . p H 7 . 5 ) t oC O l H F o rb o t h\ . r n d B t h r '
u p p e rX - r a y p r o f i l eo. f f s e tb 1 a f a c t o ro f 1 0 0 .i s t h a t o t ' t h eo r r g r n . r i
m o n o l a y etrh; e l o w e ri s t h a t a f t e rt h et r a n s f o r m a t i o int h c t a i l g r o u p
of the monolayer.
c o n t a i n i n g l 0 c a r b o n a t o m s : C l 3 S i ( C H 2 ) s C H :a n d C l 3 S i ( C (Figure 8). The fluorinatedsilaneshouldgenerate
H2)2(CF2)?CF3
a monolayerwhoseelectrondensityis closeto that of the silicon
substrate.The amplitudeof the minimum is much lower for the
fluorinatedalkylsiloxanethan for the hydrocarbon.(The positions
of the minima are different sincethe fluorinatedsilanehas two
electrondensitvregimesalong the normal axis,one for the layer
containingthc two CH2 groupsand one for the layer containing
the eight-carbonperfluorinatedchain. The alkylsiloxanemonol a y e r c o n t a i n i n gt h e ( C H 2 ) e C H 3g r o u p h a s a u n i f o r m e l e c t r o n
density throughout the monolayer.)
Monolayerscomposedof hydrocarbonhaveelectrondensities
m i d w a y b e t w e e nt h a t o f s i l i c o na n d a i r a n d a r e v e r r u m c n a b l c
t o i n v e s t i g a t i ob
n y X - r a y r e f l e c t i o n .F o r o t h c r s \ s t c m s .s u c ha :
t h e f l u o r i n a t e dm o n o l a l ' eor n s i l i c o ns h o u n i n F i g u r ef i t r r h r d r o c a r b o nm o n o l a r e r o
s n t r a n s i t i o nm e t a ls u b s t r a t eosr o n \ \ a t e r .
t h e a c q u i s i t i o no f u s e f u lr e s u l t sf r o m X - r a y r e f l e c t i v i nu i l l g e n e r a l l y r e q u i r ed e t a i l e da n a l rs i s .
Characterizationof ChemicalReactionsInrolvinga !lonolayer.
W e h a v eb e g u nt o e r p l o r et h e u s eo f X - r a l r e f l e c t i v i t vt o s t u d y
c h a n g e si n t h e s t r u c t u r r 'os f m o n o l a l ' e rw
s h e nc h e m i c a rl e a c t i o n s
a l t e r t h e i r c o m p o s i t i o n \ \ ' e h a d n v o i n t e r e s t si n t h e s es t u d i e s .
F i r s t , w e u ' i s h e dt o d e t e r m i n ei f X - r a v r e f l e c t i v i t yh a d t h e s e n sitivityto pro','ide
a ne\\ analrticaltechniquewith which to follow
r e a c t i o n si n v o l v i n gm o n o l a \ e r s . W e w e r e e s p e c i a l l yi n t e r e s t e d
i n i t s a b i l i t l t o d e t e c t s m a l l c h a n g e si n e l e c t r o nd e n s i t y ( f o r
e x a m p l et h a t a c c o m p a n v i n g
o x i d a t i o no f a C H : C H 2 g r o u p t o
a C O 2 H g r o u p ; . \ \ ' e r r e r ea l s o c o n c e r n e dw i t h i t s p o t e n t i a lt o
d a m a g et h c s a m p l ed u r i n g a n a l y s i s .S e c o n d ,w e w i s h e dt o s e e
if the structuresol the alkllsiloxanemonolayersweresufficiently
rigid and well-orderedthat we could incorporateinto them layers
h a v i n gl a r g ev a l u e so f ( d p . 1 / d z )( f o r e x a m p l e ,b y a d d i n g B r 2 t o
a C H : C H r g r o u p t o y i e l d a C H B T C H 2 B Tm o i e t y ) .
We have previouslystudied the addition of bromine to a
m o n o l a y epr r e p a r e df r o m C l 3 S i ( C H z ) 1 5 C H : C H 2( H T S ) . ' 6 T h e
contact angle of water on this vinyl-terminatedmonolayerwas
lf;'o = 100o. Reactionwith elementalbromine generatedwhat
we hypothesizedto be the correspondingI,2-dibromide(and other
relatedbrominatedspecies)78
and resultedin a decreasein dfzo
with
monolayers
terminated
Figure10. XPS spectraof alkylsiloxane
C H B T C H 2 B T( a n d r e l a t e d b r o m i n a t e ds p e c i e s i,n d i c a t e db y
*CHBrCH2Br")and CO2Hgroupsafterexposure
to X-ray radiation
source:surveyspectra(left) and high-resolution
from a synchrotron
that hadnot
spectra
of theC ls region(right): (A) edgeof monolayer
X-ray radiationand (B) centralarea
to any synchrotron
beenexposed
with the greatest
flux of X-rays.
that hadbeenirradiated
t o - l l 0 o . X P S \ p e c t r ac o n f i r n t e dt h e i n c o r p o r a t i o no f b r o m i n e
\ g g c s t c dt h a t t h e m o n o l a y ' e r
n r l t rt h c l l ( ) n ( ) l i r \ c r [ : i l i p r- r' r n t e t r\ u
h . , . i. er r g l : t"ee, l l ' ' - - I \
i r S u r c' ) f r J . e: r l . \ r . r r r c l l c , , l rirt r d r t t l if i t r t h c b r o m i n a t i o n
r l l '. r l n t r n r r i .ci f\ f . r ; s p . 1 11erd' ,.t t t. It T S R et l c et i r i t i c su c r c n l e a s u r e d
t ' r t r nlrr . i n g l c n t ( ) n \ ) i . r l cb rc l ' o r ca n d . r f t c rc x p o s u r ct o a s o l u t i o n
o l ' c l en r c n t a lb r , r n r r n rcn C H . C l . . . ' \ l - t c r c a c t i o n t. h e p r i m a r y
m i n i m u ms h i f t c dt o l o r r c rq : ( 1 r 1 ,=- 0 . 0 1 1 A - r ) . S i n c ct h e b r o m i n a t i o n e f f e c t i v c l yl e n g t h c n st h e m o n o l a y e rb y o n e a t o m i c
c e n t e r , 8t0h i s c h a n g ei s e x p e c t e da n d i s c o n s i s t e nwt i t h t h e e l l i p sometricdata. The additionof one methyleneunit to a saturated
alkyl chain containingl7 carbonatomswould shift q, by 0.0063
A - 1 . 8 r T h e i n t e n s i t i e so f t h e m i n i m a a l s o c h a n g e du p o n b r o w h i l e t h e s e c o n dd e m i n a t i o n ;t h e p r i m a r y m i n i m u m d e e p e n e d
c r e a s e di n a m p l i t u d e .
If the brominewere localizedin the positionof the doublebond
conformationfor the organicchain,we would
in a trans-extendcd
c x p e c tt o i n l - c rf ' r o n rt h e X - r a y r c f l e c t i v i t l ' al a y e ra p p r o x i m a t e l y
- 1 - At h i e k u i t h r r nc l c e t r o nd c n s i t rs e v ' c r at il n t e st h a t o f t h e h y r o d e ld i d
d r ( ) e r r r b ( ) lnr t t r n r i h c l n t c n s l t \d a t a t o a t h r e c - l a v em
( 1 8 ) B c c a u s co t ' t h e h r g h d e n s i t yo f v i n r l g r o u p s a t t h e a i r - m o n o l a y e r
r n t er f a c e . b r o m i n a t i o n o f t h e s eg r o u p s c o u l d c o n c e i v a b l yi n d u c e s o m e p o l y m e r i z a t i o n o f t h e t y p e p r e s e n t e di n e q i v .
_CH:CHZ
+ CH':611_
JA
_CHBTCH2CH2CHBT_
(iV)
(79) In using ellipsometry to follow reactions, we continued to use a refractive index of 1.4-5. This approach is clearly arbitrary, but we have found
that even using the refractive index of elemental bromine ( L66) to describe
the new layer on the surface alters our estimate of the change in thickness by
only -9.4 4.
(SO) fh. van der Waals radius of bromine (1.95 A) is slightly lessthan
t h e s u m o l t h e c o v a l e n tr a d i i o f c a r b o n ( 0 . 7 7 A ) a n d h y d r o g e n ( 0 . 3 7 1 A ) a n d
the van der Waals radius of hydrogen (1.2 A). The volume occupied by a
bromine atom is therefore similar to that of a methyl group. Lange's
Handbook of Chemistry, l lth ed.; Dean. J, A.. Ed.: McGraw-Hill: New
York.1973.
( 8 1 ) W e h a v e c a l c u l a t e d t h e e x p e c t e dc h a n g e i n q , u s i n g t h e a s s u m p t i o n
that the monolayer-air and monolayer-substrate interfaces are perfectly sharp.
In the appendix (see the supplementary material) we show that this assumption yields the usual condition for destructive interference (eq J). For
the primary minimum in R, r in equations H, I, and J is zero. Rearrangement
of equation H with n = 0 results in equation v for the location of the primary
Q,o= r/d
(v)
minimum, Qzn,ds a function oi d, the distance between the two interfaces.
Values for d were calculated using standard bond lengths and a bond angle
of 109.5o.
.Sell - .^l.ssembled
Monolayers of Alkt,lsiloxaneson Silicon
lrnd a localizedlay'erof high electrondensity. The bestfit to the
d a t a . h o w e v e r s, u g g e s t e d
a rather broadlayer (6 A (f*hm) in
thrckness)whoseelectrondensitycorresponded
to approximatel;60'7 ef that expectedfor completebrominationof the vinvl groups
in the monolayer
T h e s er e s u l t sd o n o t i n d i c a t ea w e l l - o r d e r e dl a, v e r e ds t r u c t u r e
t ' o rt h e b r o m i n a t e dm o n o l a y e rd e r i v e df r o m H T S . 8 2 T h e i r i n t e r p r e t a t i o ni s , h o w e v e r c, o m p l i c a t e db y X - r a y ,d a m a g et o t h e
b r o m i n a t e ds a m p l ed u r i n g t h e r e f l e c t i o nm e a s u r c n r c n t sb .v u n ec r t a i n t l 'c o n c e r n i n gt h c s t r u c t u r e sf o r m c do n b r o m i n a t i o nr. u r d
b r d a r n a g et o t h e s a m p l ed u r i n g t h c r e f l c c t i v i t vm e a s u r c m c n t s
b e J o r er e a c t i o nw i t h b r o m i n e . A f t e r m e a s u r e m e notf ' t h c X - r a r
reflectivityof the vinyl-terminated
monolaverprior to brominutron.
t h e c e n t r a lr e g i o no f t h e s a m p l eh a d a c o n t a c ta n s l cr i i r h r r u l c r
a p p r o x i m a t e l3
y 0 o l o w e rt h a n t h e e d g e so f t h c s a n t p i er i-t i i r \ \ c r c
o u t s i d eo f t h e X - r a y b e a m . A f t e r b r o m i n a t i o nt.h e c c n r n l rr t i ' c . L
o f t h i s s a m p l eh a d - ac o n t a c ta n g l eo i d f ; z o= 6 J o . I l o l c s rr h r r n
t h a t o f t h e e d g e( d f ; z u= 7 3 o , . F i g u r el 0 p r e s e n t X
s pS :pccrr.r
i o r t h e b r o m i n a t e dm o n o l a y e r .T h e s u r v e vs p e c t r ai n d i c a r ct h a r
t h e r ew a s o n l y o n e - t h i r da s m u c h b r o m i n ei n t h e r e g i o ne x p o s e d
[o the X-rays as in the sectionnot exposedto the radiation. The
C ls spectrawerealsoqualitativelydifferentin theseregions:the
exposedarea showedseveraldifferentcarbonenvironmentswith
b i n d i n ge n e r g i e sa t l e a s t3 e V h i g h e r t h a n t h a t o f C H r . S i n c e
thc contactangleson the surfaceof the vinyl-terminatedmonolayer
r n d i c a t e ds o m ed e g r e co f r a d i a t i o nd a m a g ep r i o r t o t h e b r o m i n a t i o no f t h e m o n o l a y e r t, h e r e d u c e dc o n c c n t r a t i o n
of bromine
that rre observedprobablyreflectsa combinationof two effects:
i i r s t . t h e r a d i a t i o nd e s t r o y e ds o m c f r a c t i o n o f t h e i n i t i a l v i n y l
- q r ( ) u pa
s .n d s e c o n dt.h e s r n c h r o t r ( ) nr a d i a t i o nr e m o v e ds o m eo f
t h c b r o n r i n et h r i th l d a d d c dt o t h e r c n r a i n i n rgi n r I g r o u p s . ss {r
F i g u r c 9 p r c s c n t sa n a l ( ) g r ) ur \el l c c t r ri t r d i r t l rt ' , i ri r l r t er i u i s
' . r b t . i r n cbdr . . t r r d l t r o n
* i r h K \ 1 n O . a n d \ l I ( ) 1 r r l n t ( ) n ( ) l ic1r \
p r e p a r e di r o m H T S T h e e r p c c t c dp r o d u c to f t h r . r c i i L t t i ) rn\ . i
c a r b o x lri c a c i d . ! 5 . \ s f o r t h e b r o n t i n a t i o n\.\ c n t c i r \ u r c -rchi e
reflecti'in from a singlcmonolarerbeforeand after reaction Thc
X - r a y d a t a i n d i c a t c da s l i g h t i n c r e a s ci n t h e t h i c k n e s o
sf thc
m o n o l a y e ro n o x i d a t i o n ,a l t h o u g ht h i s c h a n g ei n t h i c k n e s sw a s
n o t a s l a r g e( A q , = 0 . 0 0 8A - l ) a s t h a t o b s e r v e d
on bromination.
T h e s e c o n dm i n i m u m w a s a l s o r e d u c e di n a m p l i t u d ea f t e r t h e
o x i d a t i o n . S i n c et h i s r e a c t i o nr e p l a c e sa c a r b o na t o m w i t h t w o
o x y g e na t o m s ,b u t d o e sn o t a d d t o t h e e n d - t o - c n dl e n g t ho f t h e
c h a i n .w e d o n o t e x p e c t h e c h a n g ei n t h e t h i c k n e s so f t h e r n o n olaler to be as largein this reactionas in the brominationreactron.
,Attemptsto model the observeddata suggestedthat thcre was
a h i g h - d e n s i t yr e g i o n a t t h e a i r - m o n o l a y e r i n t e r f a c e . T h e
agreementbetweenthe model and the data was, however,poor.
Contact-anglemeasurements
on the vinyl-terminatednronolayer
after the determinationof its X-ray reflectivityrcveiilcdtlpicar
r a d i a t i o nd a m a g e . A f t e r t h e r e f l e c t i v i t ym e a s u r c n t c n to\ n r h e
oxidizedmonolayerthere was.however,no observablcdil'f'crcncc
i n t h e c o n t a c ta n g l e so f w a t e r Q f ; r o. = 4 0 o ) b e t u , e c nt h c r c p r u n
o f t h e s a m p l ew h i c h h a d b e e ne r p o s e dt o X - r a v s a n d t h e r c ! r ( ) n \
w h i c h h a d n o t . T h e X P S s p e c t r a( F i g u r el 0 ) a l s os h o r in , rd i l f e r e n c eb e t w e e nt h e i r r a d i a t e da n d u n i r r a d i a t e d
r e g i o n r .T h r .
a p p a r e nut n i f o r m i n i n t h e s u r f a c ea n d t h e r e s u l t i n gi n r p l i c a r r o n
t h a t X - r a y d a m a g ei s n o t i m p o r t a n ti n t h c t h e s eX - r a r r c l l c c r rrrr r
e x p e r i m e n t si s r e a s o n a b l eb u t p o s s r b l rm r s l c a d i n g B t r r l 1 1 - r .
K M n o a / N a l o o o x i d a t i o na n d t h e s r n c h r o t r o nr a d i u r i t r n
*rruitj
beexpected
to generate
o x i d i z e dr p . . , . r r n t h c r n o n o l . r r c r . . r n d
i t m i g h t n o t b e p o s s i b l ef o r u s t o d e t e c tr a d i . r r i o nd a n r ; . r srcn r h r :
o x i d i z e ds y s t e m .
( 8 2 ) w h i l e a n g l e - r e s o l v ex d
P S s t u d i e sm i g h t s h e ds o m el i s h r r r nr h c d r ' t r i b u t i o no f b r o m i n ew i t h i n t h e m o n o l a y . r *. i h u u . h a d d r i i i c - - u lonb r a r n r n p
reproducible
resultsfrom brominatedmonolayers
which had nor b.cn er|r',.cd
t o s y n c h r o t r o nr a d i a t i o n .S e er e f 1 6 .
( 8 3 ) w e h a v eo b s e r v e d
a d e c r e a s ien t h e i n t e n s i t y ' o tf h e b r o m i n cs i g n a l
d u r i n gt h e a c c u m u l a t i oonf t h e X P S s p e c t r a .w h i l e t h e f l u r o f X - r a r s i n t h c
spectrometer
is unknown,it is certainlylessthan that of thc X-rar beim irom
the synchrotron.
( 8 4 ) - T h eb i n d i n ge n e r g yo f t h e b r o m i n ei n t h e d a m a g e dr e g i o n( B r . 1 d .
_2,
7 0 . 5e V ) w a s i d e n t i c a w
l i t h t h a t i n t h e a r e a su n e x D o s etdo r h e X - r a r s .
( 8 5 ) L e m i e u xR, , U , : v o nR u d l o f f E
. . C a n .J . C h i m . l 9 5 S .J J . l 7 0 t t 7 0 9
J . . { n t ( ' h e n t .S o c . ,V ' o l .I I 1 , . \ , o I 5 l q r e
5:59
Discussion
T h i s w o r k m i r k c si t p o s s i b l et o c o m p a r em e a s u r n
e t c n t . r r l -r h e
t h i c k n e sosf a l k r l s i l o \ ; r n e
m o n o l a v e rosn s i l i c o nu s i n ct u o t c c h niques:opticalellipsontetrland low-angleX-ra.v.'
reflectirirr Thc
f o r m c rt c c h n i q u ci s m o r cc o n v e n i e nt th a n t h e l a t t e r .b u t i t . u r c
requirescertainassumptions
whosecorrectness
is difficult to chcck
T h e g o o d a g r c e n r c n tb e t u ' e e nr c s u l t sf r o m t h e s ei n d e p e n d c n t
techniques
stronglr \upp()rtsthc uccuracvof the thicknesses
from
c l l i p s o m c t r l ' . ' l - l r: er n r ril \ \ s t e r n tui c d i f f e r e n c eosb s e r v e b
de t w e e n
t h e s es e t so f r c s L r l tcsr n p h a - s r ztehsc i n t p o r t a n c eo f d e t a i l e dc o n siderationol'tlic \tructurcand properties
of the interfacesinvolved
in rcflcctrngIisht in the opticaland X-ray regionsof the spectrum.
i l l i n r ; r t c l rr h e c o r r e c t n e sosf e l l i p s o m e t rrve l i e so n t h e p r o p e r
. 1 r , , ' ' . 't , l ' l l i c i r . . ' l ' r ' , r r -i 1n rd' c. rx t r f t h e m o n o l a y e r . W h i l e t h e
' , ' , ' 1 ri' ' r q. \ , q ' I i ' . . :\ l - i r \i r n d e l l i p s o m e t r irce s u l t si s n o t
. 1 L :e. e
. L t ; l t r t j | ' t , r ( i el c r n i l n ct h r si n d e xa c c u r a t e l yw, e n o t et h a t t h e
c l c et r , r nd c n : r r o l 1 [ g n r o n o l a v ei rs a p p a r e n t l yi n d e p e n d e notf
b t r t l 1 5 . d c g r e . .)' l . ( ) r i r n l e t c n eosfst h e m o n o l a y e ar n d t h e l e n g t h
, r l ' t h cu l k r i g r , , u ni n t i r c\ l i i l n e. L . s i n gt h e s a m er e f r a c t i v ien d e x
I o r r r l ls u n r p l c .r.rl r c r h c rp . r r t i l l o r f u l l l ' f o r m e d .t h e r e f o r ea p p e a r s
l u s t i f i c d T h l ' . 1 r 1 g l g s 1 ot i1i f1' f ' e rl sr 6 m t h a t r c a c h e df o r p a r t i a l ,
" s k e l e t i z c dl"l l n t r p r e p r r r c b
d r t h c c - r c h i nogf [ . a n g m u i r - B l o d g e t t
r n u l t i l a r c r sr.; r t h e -trh . r nt h c d i r c c td c p o s i t i o n
o f p a r t i a l l yf o r m e d
m o n o l a v c r s . s t ' *I-t i s p l a u r r b l ct h l t t h r s n p c r i f m a n i p u l a t i o n
m i g h t f i c l d a n i s l r r n d\ t r u c t u r er a t h c rr h a nt h c : r p p a r e n t luyn' i f o r m
p a r t i a lm o n o l r r er s s t u d i e dh er e .
T h e i n l o r n t a t i o na v a i l a b l cf r o r n X - r a i , 'r c f l e c t i v i t 1c,o, n c e r n i n g
organicmonolaverfilms is complementaryto that availablefrom
o t h e r t c c h n i q u c s . X - r a 1 r c f l c c t i v i t y r e q u i r e sn o a p r i o r i a s s u m p t i o n sa b o u t t h c s t r u c t u r e( i n d e x o f r e f r a c t i o n ,r o u g h n e s s ,
t h i c k n c s so) l - t h c s u n t p l c I t h a s a s c n s i t i v i t lt,o a t o m i c - s c a l e
\ l : ' u ! l r ; r et h ; r lc r r n r f :u i 1 [ 1i h c s h , ) r l\ \ l t v d l e n g t ht f X - r a y l i g h t .
, - " , , : t ' . , r \ - r r l : t r ) [ r r n ! l r a t cs o l i d sm a k e si t
J r . , r l 't 1l , ' i
,;';',:t:;'.
. " i r ' ' l - t . . e \ c n r l t h c t x ' c r l r i n igi l m i s n o t
11.:l1.l.1lcl,l :l ii'.'i11..1.
.;rcLlrulll.
\ - r r r r r c l ' l c et i r r l \ . i l : r , h . r : s o c r a l l i n t i t a t i o n s . F i r s t , i t r e q u i r e s
r r s u i t r i b l r l l u t : u b ' t n r r e . \ t p r e s e n t ,h i g h l y p o l i s h e d g l a s s , f l o a t
- s l a s sa. n d ' i l i c , r n . i r c t h c o n l r s o l i d st h a t h a v e b e e n s h o w n t o h a v e
s a t i s f a c t ( ) r rl l . r l n e. r . " ' ' ' u l t h o u g h a n u m b e r o f l i q u i d s 4 e - sal n d
l i q u i d c r y ' s t a l s ' r" h ; . r v cb c c n c x a r i i n e d u ' i t h t h i s t e c h n i q u e . R e c e n t
p r o g r e s s i n t h e e p i t a x i a l g r o w t h o f m e t a l s u r f a c e s e 0a n d t h e
p r c p a r a t i o n o f u l t r a s n t o o t h s u r f a c e s e ls u g g e s t st h a t t h e e x t e n s i o n
o f t h i s t e c h n i q u e t o o t h e r s u b s t r a t e sw i l l s o o n b e p o s s i b l e . S e c o n d ,
t h e e l e c t r o n d e n s i t y o f t h e m o n o l a l c r n t u s t b e d i f f c r en r i r o r n t h a t
o f b o t h t h c s u b s t r a t ei t n d u i r : t t x r c l t r s cn t r r i c h r n gr r i t h c i t h c r r c s u l t s
i n a n i l l - d c f i n e dr n t e r t ' , i t{cl h : r t r ' . . r \ r n i i l lr l l u c o l ' r d p . 1 1 d : 2a t
t h c i n t c r l ' a c )e l tn t j , r t i c t t r , r \ r ' t n \ e r i \ t t t \i t r u n d r c s o l u t i o n .T h i r d ,
o r g a n i e s . t t n p l c .: r r r , , l r t ' r 1 ,i:l . i s e d D \ e \ p o s u r e t o h i g h - i n t e n s i t y
\ - r l r - r t I r r l r t j i , l :\ : r : j 1 i r c . cn r t r n t l l a r c r si n t h e p r e s e n c eo f d i . r \ \ S C n . t i . l ) e . : - i 'ir,l, r - e
\ r . l i 1 nt r r 1 f l 1 1 1 1 O
E nx .p O s u f eO f m O n O l a y e f s
L ) , r t . r r n r : .( 1 [ ] ' ' ' , r t t l . : . \ u l i \ i n a l o s so f b r o m i n e . T h i s t y p e
r r i 1 . : : : . . : l : , , , l : i ' i , , c d d u r i n i : X P S a n a l y s i su n d e r c o n d i t i o n st h a t
t i , n , , l , i . r : - ' , . , !: il c l h r l - r r r r i n r l - t c r m i n a t e dm o n o l a y e r s . l 6H o w
: l l , i ' . . : t l : l . - . e . i . : ; j ] . r ! un r o c e s s c a
s r e i n c a u s i n ga r t i f a c t s i n t h e
' i . , t . : . r r t t l t , , * r i l c u t r r c l r t h e y c a n b e s u p p r e s s e db y c h a n g i n g
r lo n d r t i o n s( f o r e x a m p l e , b y u s i n g i n e r t a t m o s p h e r e s
e\ , i ' \ c : t n r c n l . e
r , l \ . i \ - u r . t n tl (. ) \ \ t c n l p c r a t u r e s ,o r s h o r t e x p o s u r et i m e s ) r e m a i n s
t ( ) i , ! -c \ t . r b l t ' h c d \ \ ' c b e l i e v et h a t b c t t c r c t t n t r o l o v e r t h e c o n d i t i o n s
r . : r t j c -rrr h r c h X - n r , r r c f ' l e c t i r i t l m e a s u r e m e n t sa r e m a d e w i l l p e r m i t
l i r c u s c o l ' t h i s t c c h n r q u c f o r t h e d e t a i l e d a n a l y s i so f t h e s t r u c t u r e
, r l -n r r r n o l a l c rs \ s t c m s .
Experimental Section
\ l a t e r i a l s . D e c y l - ,d o d e c y l - ,r e t r a d e c v l - h
. e x a d e c v l -a, n d o c t a d e c y l trrchlonrsiianw
e e r e o b t a i n e df r o m P e t r a r c hS y s t e m sa n d d i s t i l l e dp r i o r
( 8 6 ) B l o d g e t tK, . B . : L a n g m u i r l. . P h t , . rR. c r ' .1 9 3 7 .5 1 , 9 6 4 - 9 8 2 .
( 8 7 ) T o m a r ,M . S . J . P h y ' s(.' h e m .1 9 7 4 .7 8 , 9 4 7 - 9 5 0 .
( 8 t i )C o w l e y R
. . A . : R y a n .T . W . J . P h y s .D 1 9 8 7 , 2 0 , 6 1 - 6 8 .
(89) Materialssuchas cleavedmica or graphitemay havesuitableflatness
f o r t h e a p p l i c a t i o no f t h e X - r a y r e f l e c t i o nt e c h n i q u eb, u t t h e y h a v en o t y e t
beendevelopedfor this prupose.
( 9 0 ) H a l l m a r k ,V . M . : C h r a n gS
, . : R a b o l t J. . F . ; S w a l e nJ, . D . ; W i l s o n .
R. J. Ph,r's.Rec.Lett. 1987..t9. 28'/9-2882.
( 9 1 )B r o w nN
, . . 1 . . 4 n n uR
. e r .M a t e r .S c i . 1 9 8 6 ./ 6 . 3 7 1 - 3 8 8 .
5860
J. ,4m. ('hent. Soc.. l'ttl. i I l. ,\'o. 15. lA89
Wasserman et al.
t o u s e , T h e c o n r p o u n d- r , - 1 . { . { . - i . - i . 0 . o . 7 . 7 . t J . 8 . 9 , 9 , 1 0 . 1 0 , 1 0 - h e p t a d esct rae-a mo f a r g o n .a n d i n t r o d u c e di n t o t h e s p e c t r o m e t e r .F o r e a c hs a m p l e
f l u o r o d e ol t r i c h l o r o s i l a nrcC i r S r (C H , ) : (C F 2 ) i CF j ) w a s o b t a i n e df r o m
a s u r v e vs p e c t r u m( r e s o l u t i o nl . l e V , s p o t s i z e 1 0 0 0i r m , o n e s c a n ) a n d
P e t r a r c ha n d u s e d a s r e c e r v e d .T h e s l ' n t h e s i so f l 6 - h e p t a d e c e n y l t r i h i g h - r e s o l u t i osnp e c t r ao f t h e p e a k sf o r C l s , O l s . B r 3 d , a n d S i 2 p
( H T S ) h a s b e e nd e s c r i b e dp r e v i o u s l y . r 6H e x a d e c a n e
chlorosilane
( r e s o l u t i o n0 . 1 6 c V . s p c ' rst i z e 3 0 0 p m , l 0 - 3 0 s c a n s ) w e r e c o l l e c t e d .
and
b i c y c l o h e r r lr r c r e r ) b t a i n c df r o m A i d r i c h a n d p u r i f i e d b , vp- .e r c o l a t i n g
A t o m i c c o m p o s i t i o nw
s e r e d e t e r m i n e dw i t h s t a n d a r dm u l t i p l e xf i t t i n g
t w i c et h r o u g hn eu t r r i l .c r r d c l . u c r i r a t e d( a s p u r c h a s e da) l u m i n a( F i s h routinea
s n d t h c f o l l o w i n gs e n s i t i v i tfya c t o r s :C l s , 1 . 0 0 ; O 1 s , 2 . 4 9 ; S i
'r'
e r ) . T h e p u r i l r c d s o l re n t : p a : s e dt h e B i g e l o wt e s t f o r p o l a r i m p u r i t i e s . e 2
l p . 0 . 9 0 :B r l d . 1 l s S
S i l i c o n( 1 0 0 )* a s o b t a i n e di n 3 - i n .d i a m e t e rw a f e r sf r o m S e m i c o n d u c t o r
X-ray' Reflection Measurements. X-ray sourceswere a Rigaku roP r o c e s s i nC
g o r p t B o s t o n .\ 1 { ) ( n - t y , p el .a s e rg r a d e )i n t h r e e t h i c k n e s s e s ,
t a t i n g - a n o d(eR A ) X - r ; n g e n e r a t o(rC u K a 1 r a d i a t i o n)., = 1 . 5 44 . 9 0
0 . 0 8 0 .0 . 1 1 5 .u n d 0 1 0 0 r n . . a n d f r o m M o n s a n t o( p - t y p e .0 0 1 5 i n . ) .
m A . 4 5 k c \ ' ) u n d t h c \ . r t i o n a l S r n c h r o t r o nL i g h t S o u r c e( N S L S ) a t
W a t e r \ \ a 5p a s s c dt h r t r u g ha n i o n e x c h a n g e(rC o l e - P a r m e ra) n d d i s t i l l e d
B r o o k h a v c n\ i r t i o n r r l [ . l b t i r a t o r ] ( b e a m l i n e X - 2 2 8 , \ = l . 7 l A ) .
i n a ( - o r n r n g\ l o d c l \ ( i - l b g l a s sd i s t i l l a t i o a
npparatus.
M o n o c h r o r n l t r cr a d i u t r t ) n\ \ a s o b t a i n e db v r e f l e c t i o nf r o m a m o n o c h r o P r e p a r a t i o no f \ l o n o l a r e r s . T h e s i l i c o nw a f e r sw e r e c u t i n t t l s t r i p s
m a t o r ( R A . t r r p l c - b o u n cgec r m a n i u n r( l I l ) : N S L S , s i n g l e - b o u n cgee r l - r n u i d c T h c r c . t r r p su e r c c l e a n e db y h e a t i n gi n a s o l u t i o no l c o n m a n i u m( l l l ) ) . T h c b c a ms i z er . r ' a0s. I X 5 m m f o r i n c i d e n a
t n g l e sl e s s
c e n t r a t c dt { . S O . 1r i n d - 1 0 ? H 2 O 2 ( 7 0 : 3 0v f v ) a t 9 0 o C i o r 3 0 m i n . e 3
t h a n l o a n d 0 . 5 X 5 m m f o r i n c i d e n ta n g l c sg r e a t e rt h a n l o . X - r a y s w e r e
'
p
i
r
a
n
h
o
(CAt.TIO\
solutionreactstittlently with many organit
m o n i t o r e dw i t h t u ' o s c r n t i l l i r t i o nd e t e c t o r s :o n e f o r t h e i n c o m i n gb e a m ,
m a t e r r a l \ u t t d . t h , t u l dh e h u n d l e dw , i t hg r e a t c a r e . ) T h e s u b s t r a t e sw e r e
t h e o t h e r f o r t h e r a d i a t i o nr e f l e c t e df r o m t h e s a m p l e . T h e i n t e n s i t i e so f
r i n s c dt h o r . . u g h l r* i t h d i s t i l l e dw a t e r a n d s t o r e du n d e rw a t e r u n t i l u s e .
t h e r c l - l e c t eX
d - r a v s u ' e r en o r m a l i z e dt o t h e i n t e n s i t yo f t h e i n c o m i n g
T h c c i c a n e d: r l i c o n \ t r i p \ u e r e r e m o v e df r o m w a t e r u s i n g T e f l o n beam.
c o a t e df o r c e p s( P e l c o ) . A l l v i s i b l et r a c e so f w a t e r w e r e e l i m i n a t e db y
S i n c e t h c b a c k g r o u n dr a d i a t i o n w a s a f u n c t i o n o f t h e a n g l e o f t h e
e x p o s r n gt h e s a m p l ct o a s t r e a mo f a r g o n ( m i n i m u m p u r i t y 9 9 . 9 9 5 % )i o r
i n c o m i n gb e a m . p o i n t b l p o i n t b a c k g r o u n ds u b t r a c t i o nw a s p e r f o r m e d .
- - 1 0 s T h e s i l i c o nr . r a st h e n p l a c e di n a - 0 . 5 V c w / w s o l u t i o no f t h e
T h e b a c k g r o u n dw a s d c t e r m i n e db y p u r p o s e l ym i s a l i g n i n gt h e d e t e c t o r
a l k r l t r i c h l o r o s i l a nien h e x a d e c a noer b i c y c l o h e x y ' lT. h e c o n t a i n e r sf t t r
b r * 0 . J o e t c l r c hi n c i d e n at n g l cH
t h e s o l u t r o nw e r ec u s t o m - m a d e
f r o m r e c t a n g u l a gr l a s st u b i n g t h l t h a d
i l t b r l r s sc c l l * r t h K a p t o n( D u P o n t )u i n S l t r r t i ' l c\ s\ c r c l t t ( ) i l n l c rt n
o n ee n d s e a l e d .P r i o r t o u s ea n d d u r i n g t h e f o r m a t i o no f t h e a l k r l s r l o r a n e
t l r r u r l h c . i r . i n l ) r ' r( ' \ r ' i r i ( i c\d- r - . r t \ J l a n g l e sg r e a t e rt h a n 7 o T h e
m o n o l a l e r st.h e c o n t a i n e r w
s e r ek e p t e i t h e ru n d e rI d r r n i t r , r u c na l r n , r l i l i | { r \ n h c r -' r ' r , r ' . i t . r l t , b \c\ r: i \ c r t h c rl t r rt t r h c l i u m .
' ' l i r r ' r l ' r ' .l l . i t u r r r r l b
s p h e r eo r i n a d e s i c c a t ocr o n t a i n i n gP z O s( B a k e r ,* g r a n u s i c ' ) . \ f ' t c r l
i r ' er , r r '
r ie d c t c c t c du a s l 0 b u i t h t h e r o h ( d e s i c c a t o ro\ r 2 4 h ( n i t r o g c na t m o s p h e r c )t.h e s u b s t r l t e\ \ a \ r en r r ) c\ ( l
1 . 1 1 , : r ! . 1 y 'I, r, , 1. i rqt ' , r 1\ \ l \
\ t r p i c r trl c l l c c t i o sn c a nr e q u i r e dl 5 h
' , r : i : i ' ' , : . : :, r r r ' r " r - i . . l r ti j h . r t \ S I S T h c d a t a t h a t w a so b t a i n e da t
f r o m s o l u t i o na n d p l a c e di n 1 0 0m L o f C H C I r f o r l 5 n r i n r ( \ r c n r ( ) \ c . r n \
m i c r o s c o p icco n t a m i n a n t tsh a t m i g h t h a v ce d s o r b c dr ) n t r t) h c . u r l - . r c,e, '
\ : l \ ! , , \ i ' i ' , : ' . r ' , - t t l r c r , l l ! t c r n q - i i \ t h t r t f r o n t t h e r o t a t i n ga n o d e .
t h e m o n o l a y c r .T h c s a n t p l cw a s t h e n i n t r n res e di n l ( ) ( )n t l i r l c i i r . r n , , l
l l r o m i n a t i o n . T i r e\ - r . l r e l l c c t i r i t r f u r a m o n o l a y e rp r e p a r e df r o m
f o r 3 0 s a n d r i n s e d* i t h c t h a n o ld i s p c n s efdr , t n ri r l - r n I d r ' p , r : . r b lper p c i
l l TS
n t ( i i \ ' , i r . d. r \ i r b ( ) \ ' eT, h i s m o n o l a ) , ewr a s t h e n p l a c e di n a 2 V o
".r\
( b r r L r l u n r e: )r r l u t r r ronf e l e m e n t abl r o m i n ei n C H 2 C I , f o r 7 h . T h e w a f e r
T h c m o n o l a \ e r\ \ a s d r i e d u n d e ra 5 t r e a m( ) l ' a r g ( ) rnr n dn t c . i , u r c n t c n (l \) l
c o n t a c ta n g l ea n d c l l i p s o n t e t r u
r ere madeimmediatcir
r r a s t h c n r r n s c dr n C l l 2 C 1 2a n d i n e t h a n o l . T h e r e f l e c t i v i t yw a s t h e n
C o n t a c tA n g l e s . A d v a n c i n gc o n t a c ta n g l e su e r e d e t c r r n r n e d
on scssilc
m c a s u r c da g a i n .
d r o p sw i t h a R a m e - H a r t M o d e l 1 0 0c o n t a c ta n g l eg o n i o m e t e re q u i p p e d
Oxidation. As for the bromination. the reflectivitiesbefore and after
w i t h a c o n t r o l l e d - e n v i r o n m ecnht a m b e r . T h e r e l a t i v eh u m i d i t y i n t h e
oxidation wcre measuredas describedabove. Stock solutionsof KMnOa
c h a m b e rw a s m a i n t a i n c da t > 8 0 % b y f i l l i n g t h e w e l l s o f t h e s a m p l e
( - 5m M ) . N a l O a ( 1 9 5 m M ) , a n d K 2 C O 3( 1 8 m M ) i n w a t e r w e r e p r e c h a m b e r w i t h w a t e r . T h e t e m p e r a t u r ew a s n o t c o n t r o l l e da n d v a r i e d
p a r e d . I m m e d i a t e l vp r i o r t o t h e o x i d a t i o n ,l 0 m L o f e a c h o f t h e s e
f r o m 2 0 t o 2 - 5o C . T h e v o l u m e o f t h e d r o p u s e d w a s 3 p L : i t s p H w a s
s o l u t i o n sw a s c o m b r n e dw i t h 7 0 m t . o f d i s t i l l e dw a t e r t o c r e a t et h e o x - 5 . 6 . A l l r e p o r t e dv a l u e sa r e t h e a v e r a g eo f a t l e a s tf o u r m e a s u r e m e n t s
r d i z i n gs o l u t i o {nK \ { n O . , . 05 m \ ' l : \ u l O a . l 9 5 m M : K " C O 1 .1 . 8m M .
o n t h e f i l m s u r f a c ea n d h a v ea m a x i m u m r a n g eo f * 3 o .
p l f - i t I h er r , ) n { ) l , r \n
r rr e p a r c (l rl o n t t l - I S * r r s p l a c e di n t h i ss o l u t i o n
' , r rI ] rr l - ' r
E l l i p s o m e t r y .E l l i p s o r n e t r ircn c a s u r e m c n t\ s4 ' e r em a d eu i t h r t R r i d r r l p h
l l ' e . i : i ' n l r ' \ \ . i r\ c l - n ( ) \ ' fcr do n tt h e o x i d a n ta n d r i n s e d
' . ' r r r r l'
ResearcM
h o d eI 4 1 6 0 . 1 - : 0 0tFh i n - f i l n cr l l i n s t r n t e r cl r' h c l r r r h \t \ ) r i r r e\ \ . i \
. . , . t r, , ! \ . , 1 1 \ O ,r t t I \ 1 ) . w a t c r . 0i \ H C l . w . a t e ar .n d
-rlrr:
a H e \ c l l r s c{rI - h l l \ \ l T h c . r n t l c , , ltn L t d L : n\ \\ ',e1 r
rrc].rll\c
.il',].'.ilrrl
t o t h e n o r r n a ol l ' t h e p l . r n co i t h e r . i n t p l c .t i n d t h c ! r ) n t l c n \ r t \ ) \i \- . i \ \ c l
P e n t a d e ol t r i c h l o r o s i l a n eD
. i h l d r o g c n h e x a c h l o r o p l a t i n a tIeI )( ( A l f a ,
a t - . 1 5 , 0 o .T h c r t t c i . t \ u r c r l t cnncl \L c \ \ r r \ l o r t h c c a i c u l r t l r )rnr l ' t h ei rl n t
- i - l n r I o l r i ( ) 0 ] \ l s o l u t i o ni n T H F , 0 . 0 5 3 m m o l ) , t r i c h l o r o s i l a n(eP e t h i c k n c s cs o n s i : t c dr , r f ' t h de e t c r n t r n a t i ooni t h c p o l a n z c r ; . r nadn a l r z e r
t r a r c h . 8 . 6 n r L - . 8 , 5m m o l ) . a n d l - p e n t a d e c e n(eA l d r i c h , 1 5 . 0 1g , 7 l
a n g l e si o r t h e : r l i e t r n5 u b s t r a t ea n d t h e c o r r e s p o n d i nsgc t o l a n g l c sl o r
m m o l ) w e r c p l a c e d u n d e r a r g o n i n a d r y , h e a v y - w a l l e dg l a s st u b e ( d i t h e s u b s t r a t ec o l r e d * i t h a r n o n o l a yre f i l m .
a m e t e r2 . 5 c m , l e n g t h2 l c m ) e q u i p p e dw i t h a s i d e a r ma n d a 0 - 1 0 m m
E a c h s e t o f a n a i r z e ra n d p o l a r i z e r e a d i n g sn. t e a s u r e idn z o n e sI a n d
P T F E s t o p c o c k . T h e s o l u t i o nw a s d e g a s s e d( f r e e z e - p u m p - t h a w ,t h r e e
3 , e aw e r e t h e l r r e r . r s eo f a t l e a s t f o u r m e a s u r c m e n t st a k e n a t d i f f e r e n t
c y c l e s )a n d t h e t u b e w a ss e a l e du n d e rv a c u u ma t - I 9 5 o C . T h e t u b e w a s
l o c a t i o n s( s e p a r a t c db r a t l e a s t I c m ) o n t h e s a m p l e . T h e a n g l e st h a t
t h e n w a r m c d t o r o o m t e m p e r a t u r e ,a f t e r w h i c h i t w a s h e a t e di n a n o i l
c o m p r i s e dt h i s a r e r a s eh a d a m a x i m u ms c a t t e ro f * 0 . l 5 o . T h e s em e a b a t h ( 9 9 " C , 4 3 h ) . T h e t u b e w a s t h e n c o o l e dt o r o o m t e m p e r a t u r e .T h e
s u r e m e n tu
s c r e d e l c r m i n e di n a i r f o r t h e b a r es u b s t r a t ew i t h i n 5 m i n o f
r e a c t i o n s o l u t i o n w a s t r a n s f e r r e dt o a 1 0 0 - m L r o u n d - b o t t o m e df l a s k
i t s r e m o v a l f r o n r * e t c r T h c s u b s t r a t ew a s p l a c e di n t h e s o l u t i o no f
e q u i p p e dw i t h a v a c u u m a d a p t e r . A l i q u i d n i t r o g e n c o o l e d t r a p w a s
a l k y l t n c h l o r o sl ai n c r m m c d i a t e l la f t e r t h e s em e a s u r e m e n t sM
. easurea t t a c h e da n d t h e e x c e s st r i c h l o r o s i l a n ae n d T H F w e r e r e m o v e db y a
-fhc
m e n t sf o r t h c r u b : t r l t e - r n o n o l a t e rs y s t e m sw e r e t a k e n n o m o r e t h a n 5
t r a p - t o - t r a pd i s t i l l a t i o n
r c m a i n i n gl i q u i d w a s d i s t i l l e di n a d r y
m i n a f t e r t h c r . i n r p i e sh a d b e e nu ' a s h e dw i t h e t h a n o l .
K u g e l r o h rd i s t i l l a t i r r ;nr p p u n r t u sT h e p r o d u c t( 1 5 . 3g , 4 4 m m o l , 6 2 V c )
The refractrrc rnder trf the substratewas calculatedfrom the analyzer
w a s t h c f r a c t i t r ne , r l l c e t ctd' r o m9 5 ' C ( 0 . 0 1 3T o r r ) t o 1 0 5 ' C ( 0 . 0 1 0
a n d p o l a r i z e ra n g l e si o r r h e u n c o a t e ds i l i c o n .T h i s v a l u ew a s t h e n u s e d
Torr).
' H \ \ , t R i ( I ) ( i , r , i l i - 1 . 2 ( m , 2 8 ) , 0 . 9( t , 3 ) . ' 3 CN M R ( C D C I 3 ) :
t o d e t e r m i n et h c t h r c k n e sos f t h e m o n o l a y e a
r c c o r d i n gt o t h e a l g o r i t h m
o f M c C r a c k i n : e T h e l e n g t h sw e r ec a l c u l a t e da s s u m i n gt h a t t h e m o n o 1 t 1 . 1 .r t o t . r 0 0 0 . t 9 9 4 , 2 9 . 9 9 ,2 9 . 6 8 , 2 9 . 2 5 , 2 4 . 5 0 , 2 2 . 9 9 , 2 2 . 5 1 ,
. \ n l l ( l l c d f o r C r 5 H 3 r C l 3 S iC
l a y e r h a d a r e f r a c t r v ei n d e xo f 1 . 4 5 . T h e a l g o r i t h mc a l c u l a t e dt w o v a l u e s
14lq
: , 5 2 . 0 8 1H , 9 . 0 5 ;C l , 3 0 . 7 5 .
f o r t h e l e n g t ho f t h e m o n o l a v e r b
. o t h o f w h i c h w e r e c o m p l e x . S i n c et h e
l r o u n d ( ' . 5 I r 9 : I { . 9 . 1 2 :C l . 3 0 . 9 5 .
l e n g t h o l t h e m o n o l i t \ e rm u s t b e r e a l , w e c h o s et h e r e a l p a r t o f t h c
c o m p l e xn u m b c r* i t h t h e s m a l l e ri m a g i n a r yc o m p o n e nat s t h e t h i c k n c s s
.{cknorrledgment. This research was supported by the Office
o f t h e m o n o l a re r t T h e o t h e r c h o i c ew a s i n h e r e n t l yu n r e a s o n a b lsei n ec
of- \avul Rcsearch. the Defense Advanced Research Projects
i t w a s g r e a t e rt h a n l t l 0 0 A . t T h i c k n e s s edse t e r m i n e di n t h i s w a \ a r e
.,\gen* (through the University ResearchInitiative). the National
a c c u r a t et o + : . 1 ,
Science l*oundation (Grant DMR 86-14003 to the Harvard
X - r a y P h o t o e l e c t r o nS p e c t r o s c o p y .T h e X P S s p e c t r aw e r e o b t a i n e d
Materials Research Laboratory). and the Joint Services Electronics
u s i n g a S u r f a c e S c i e n c e L a b o r a t o r i e sM o d e l S S X - 1 0 0 s p e c t r o m e t c r
Program of the Department of Defense (Grant N0014-84-K( m o n o c h r o m a t i z e dA l K c - rX - r a y s o u r c e ;l 0 - 8 - 1 0 - eT o r r ) r e f e r e n c e dt o
0465). XPS spectra were obtained with instrumental facilities
Au 4f112
a t 8 4 . 0 e V . S a m p l e sw e r e w a s h e dw i t h e t h a n o l ,d r i e d u n d e r a
purchased under the DARPA/URI
program and maintained by
( 9 2 ) B i g e l o wW
. . C.; PickettD
. . L . ; Z i s m a n ,W . A . J . C o l l o i d .S c i . 1 9 4 6 ,
1 , 5r 3 - 5 3 8 .
(93) PintchovskF
i , . ; P r i c e ,J . B . ; T o b i n , P . J . ; P e a v e yJ, . ; K o b o l d ,K . " I .
Electrochem.Soc. 1979, I 26, 1428-1430.
(9a) Seeref 29 for the definitionsof the angularzonesusedin ellipsometry.
(95) TheseXPS sensitiviries
are thosesuppliedby SurfaceScienceLaboratoriesin their escn 8.0B software. Thesevaluesare thosecalculatedby
Scofield,correctedfor the dependence
of the mean free path of an electron
on its energy. Scofield,J. H. "/. ElectronSpectrosc.1976.8. 129-13]..
5 8 6l
the Harvard UniversityMRL. The rotating-anode
facility is part
of the MRL. The synchrotronmeasurementswere carried out
at the National SynchrotronLight Source(NSLS) ar Brookhaven
National Laboratory. Researchat the NSLS is supportedby the
O f f i c e o f B a s i c E n e r g y S c i e n c e sU, . S . D e p a r t m e n to f E n e r g y ,
u n d e r c o n t r a c t D E - A C 0 2 - 7 6 C H 0 0 0 1 6 . I a n T i d s w e l lw a s t h e
recipientof a NATO studentship.We are grateful to Dr. David
Osterman and Dr. Thomas Rabedeaufor assistancein these
e x p e r i m e n t st,o D r . A b r a h a m U l m a n o f E a s t m a n K o d a k l o r
providingpreprintsof articles,and to Dr. Ralph Nuzzo of AT&T
B e l l L a b o r a t o r i e sf o r h e l p f u l d i s c u s s i o n s .
R e g i s t rN
y o . t { T S . 1 2 0 9 0 - s - 0 9S- il ,: 7 4 4 0 - 2 1 - 3
C:l 3 5 i ( C H 2 ) r 4 C H 3 ,
-5; Cl3Si(C
4-1: Clrsi(C H2)eC
60592-1
Hr. I 3829-21
H 2)rrC Hr, 4484-7
2-4:
C l 3 s i ( C H 2r)C' H , , | 8 4 0 2 - 2 2;-C
7l3Si(C
H 2 ) , r C H r5, 8 9 4 - 6 0 -C
0 :l 3 S i ( C H 2 )r7 CH 3 ,112 - 0 4 - 9C: l r s i ( C H 2 ) 2 F
( C2 ) 7 C F738,5 6 0 - 4 4 - 8 .
SupplementaryMaterial Available: An appendixoutlining the
intensityof reflectedX-rays for certainelectron-density
profiles
(4 pages).Orderinginformationis givenon any currentmasthead
page.