Current Electricity
advertisement

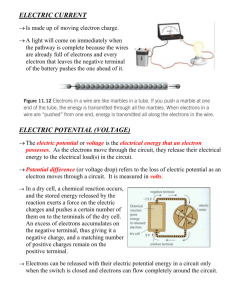
Current Electricity Nov 21­3:19 PM Electric Current A CIRCUIT is a controlled path through which electrons flow. It's made up of 4 main parts: 1. A Source of Electrical Energy­ Like a battery or solar cell 2. Electrical Load­ What uses the electricity. For example, a light bulb or computer. 3. Circuit control device­ something to turn the power on and off. 4. Connectors­ conductors like wires that create the pathway. A closed circuit­ electrical energy can flow. The load is on and can work (ie. a light comes on). An open circuit­ electrical energy cannot flow. The load cannot work as electricity is not being supplied to it. (ie. light is off) Nov 21­3:23 PM 1 Circuit Diagram A Symbolic representation of a real circuit Electricity flows from the negative terminals to the positive terminals. Nov 21­3:25 PM Answers 1. Explain the difference between static electricity and an electric current. Static electricity: doesn't move, electrons are stationary. Electric current: Electrons move. 2. Part of the circuit: Function: What part of our conductivity tester is represented by this part of the circuit? Source of Electrical Energy Provides a source of energy Battery Electrical Load What uses the energy. Light bulb Electric Circuit Control Device~: Controls the flow of energy in the circuit. Paper clip Connectors~: Connects all of the parts of Wires the circuits so electrons can flow Nov 21­3:33 PM 2 1. Explain the difference between an open circuit and a closed circuit. Closed: All of the parts are connected and electrons can flow freely through the circuit. The device would be on. Open: Something is not connected so the electrons cannot complete the circuit. The device is off. 2. In what direction does the electric charge flow around the circuit in figure 1 (page 300)? What causes it to happen? The current flows from the negative end through the circuit to the positive end. The metal used on the ends of the battery determines which end is positive and negative. Nov 21­3:39 PM Nov 21­3:52 PM 3 Nov 21­3:53 PM Nov 21­3:53 PM 4 10.3 Voltage­ Electrical Potential Voltage or Electrical Potential ­the amount of energy each electron has. Electrons at the generators in power stations have a high amount of energy (a lot of volts­550 volts), Wall outlets in your house have 120 or 240 volts. A battery (electrochemical cell) has 1.1 to 2.9 volts of electrical energy. Page 303 1,2,4 1. The electrons must move continuously around the circuit to continually provide electrons from the circuit to the positive terminals of the dry cell for chemical reactions to occur in the dry cell. b) Electrons flow from the negative terminal. 2. a) The energy each electron has as it leaves the negative terminal of the dry cell. b) Volt V 4. The electric potential energy of the electrons at the negative terminal of a 120 volt source is 20 times greater than the electrons at the negative terminal of a 6 volt battery. This extra energy is enough to deliver a severe shock. Nov 21­3:55 PM An Electochemical cell or battery : Is is portable electrochemical energy which is transformed into electric power as soon as the cell is inserted in an electric circuit and discharges. How does a cell work? There are two points : a positive terminal, the cathode and a negative terminal, the anode. Each one is made of a different metal. The anode is made of manganese dioxide and the cathode is made of zinc. Between these two points there is an electrolyte that allows ions to circulate. A chemical reaction occurs between the electrolyte and the negative anode. The chemical energy released seperates electrons, these electrons collect on the negative terminal of the cell. At the same time positive charges collec on the positive terminal. Cells discharge only when connected to a closed electric current. Nov 21­3:54 PM 5 Nov 21­3:54 PM 1. Explain the difference between a primary cell and a dry cell. In a primary cell chemical reactions use up some of the materials in the cell as electrons flow from it. Rather than the electrolyte being a liquid, in a dry cell it is a moist paste. 2. Explain why primary dry cells were developed. Primary Dry cells were developed to overcome the problems caused by the use of liquids in the voltaic cell. 3. Why shouldn’t you try and recharge a primary cell or throw one on a fire? If you tried to recharge a primary cell, the energy supplied by the charging device would cause the cell to heat up and could explode. For the same reason you shouldn't put it in a fire. 4. What are some of the ways manufacturers ensure you do not install batteries incorrectly? (You may need to look at some of your devices to figure this out). Often the shape of the device and battery is such that you can only insert it the right way. Also, some are labelled. Nov 21­3:54 PM 6 Nov 21­3:54 PM Nov 21­3:54 PM 7 Nov 21­3:54 PM 8