Topics – Gases • Gas Laws o Boyles Law, PV = K or P 1V1 = P2V2 o
advertisement
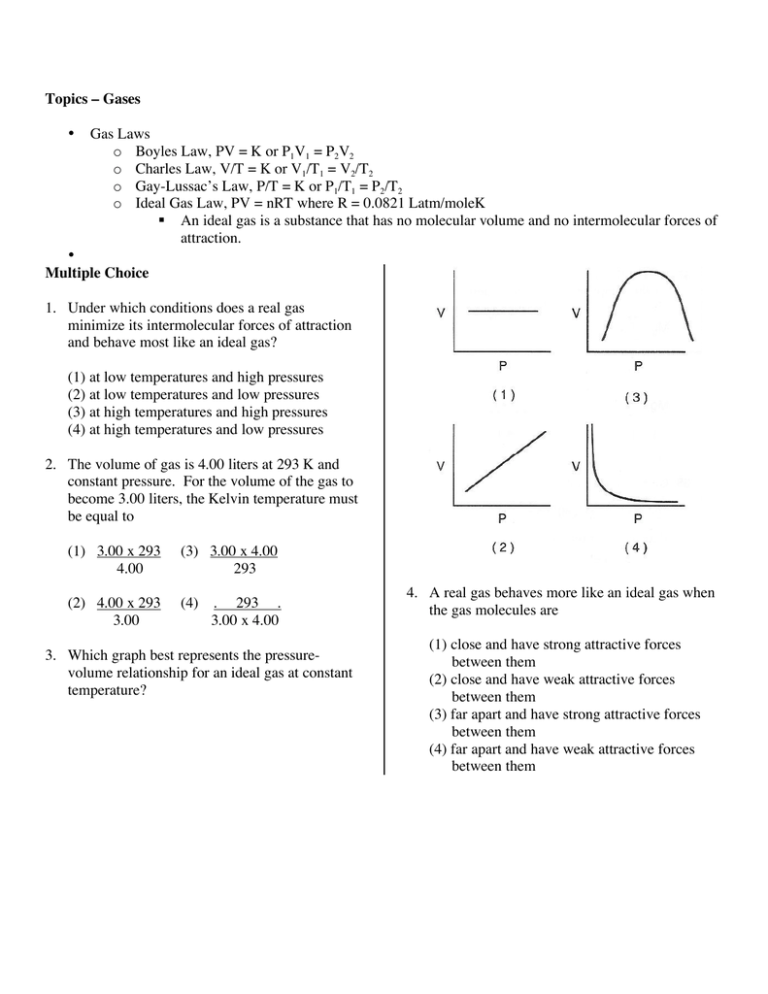
Topics – Gases • Gas Laws o Boyles Law, PV = K or P1V1 = P2V2 o Charles Law, V/T = K or V1/T1 = V2/T2 o Gay-Lussac’s Law, P/T = K or P1/T1 = P2/T2 o Ideal Gas Law, PV = nRT where R = 0.0821 Latm/moleK An ideal gas is a substance that has no molecular volume and no intermolecular forces of attraction. • Multiple Choice 1. Under which conditions does a real gas minimize its intermolecular forces of attraction and behave most like an ideal gas? (1) at low temperatures and high pressures (2) at low temperatures and low pressures (3) at high temperatures and high pressures (4) at high temperatures and low pressures 2. The volume of gas is 4.00 liters at 293 K and constant pressure. For the volume of the gas to become 3.00 liters, the Kelvin temperature must be equal to (1) 3.00 x 293 4.00 (3) 3.00 x 4.00 293 (2) 4.00 x 293 3.00 (4) . 293 . 3.00 x 4.00 3. Which graph best represents the pressurevolume relationship for an ideal gas at constant temperature? 4. A real gas behaves more like an ideal gas when the gas molecules are (1) close and have strong attractive forces between them (2) close and have weak attractive forces between them (3) far apart and have strong attractive forces between them (4) far apart and have weak attractive forces between them 5. A gas occupies a volume of 40.0 milliliters at 20°C. If the volume is increased to 80.0 milliliters at constant pressure, the resulting temperature will be equal to (1) 20°C x 80.0 mL 40.0 mL (3) 293 K x 80.0 mL 40.0 mL (2) 20°C x 40.0 mL 80.0 mL 40.0 mL (4) 293 K x 80.0 mL 6. At constant temperature, the relationship between the volume (V) of a given mass of gas and its pressure (P) is (1) V = kP (2) P = kV (3) PV = k (4) V/P = k 7. A mixture of gases has a total pressure of 2000 torr. The mixture contains 8 moles of nitrogen gas and 2 moles of oxygen gas. What pressure is exerted by the oxygen gas molecules? (1) 200 torr (2) 400 torr (3) 2000 torr (4) 4000 torr 8. As the temperature of a given sample of a gas decreases at constant pressure, the volume of the gas (1) decreases (2) increases (3) remains the same 9. The pressure on a 200 milliliter sample of CO2 (g) at constant temperature is increased from 600 torr to 1,200 torr. What is the new volume of the gas? (1) 100 mL (2) 300 mL (3) 400 mL (4) 600 mL 10. What is the volume, in liters, of 576 grams of SO2 gas at STP? (1) 101 L (2) 202 L (3) 216 L (4) 788 L 11. What is the total volume occupied by 132 grams of CO2 (g) at 0°C and 1.0 atm of pressure? (1) 22.4 L (2) 33.6 L (3) 44.8 L (4) 67.2 L 12. At the same temperature and pressure, 1.0 liter of CO (g) and 1.0 liter of CO2 (g) have (1) equal masses and the same number of molecules (2) different masses and a different number of molecules (3) equal volumes and the same number of molecules (4) different volumes and a different number of molecules 13. A volume of air is in a container whose volume can be changed using a piston. As the piston reduces the volume of the air, the number of gas molecules (1) decreases (2) increases (3) remains the same 14. What will be the new volume of a 1.00 mole sample of a gas at STP if the pressure remains constant and the Kelvin temperature is halved? (1) 11.2 L (2) 22.4 L (3) 33.6 L (4) 44.8 L 15. At the same temperature and pressure, which sample contains the same number of moles of particles as 1 liter of O2 (g)? (1) 1 L Ne (g) (2) 2 L N2 (g) (3) 0.5 L SO2 (g) (4) 1 L H2O (l) 16. A gas occupies a volume of 40.0 milliliters at 20 °C. If the volume reduced to 20.0 milliliters at constant pressure, the resulting temperature must be (1) 10 °C (2) 0 °C (3) 313 °C (4) –127 °C 17. Which graph shows the pressure-temperature relationship expected for an ideal gas? 19. Which of the following represents standard temperature and pressure? (1) 760 torr and 0 K (2) 1 torr and 0 K (3) 760 torr and 0 °C (4) 1 torr and 0 °C 20. The height of a mercury barometer on a day when the pressure was 0.938 atm is (1) 760 mm (2) 712 mm (3) 12 mm (4) 772 mm 21. Which of the following represents Boyles’ law, which is the pressure volume relationship of an ideal gas? (1) (1) PV = k (2) P/T = k (3) V/T = k (4) PV/T = k 22. Which of the following gases is most like an ideal gas? (1) He (2) Ne (2) (3) Ar (4) Xe 23. If the temperature of a gas is held constant and the pressure doubles, the volume of the gas must (1) double (2) halve (3) remain the same. (3) 18. What is the temperature of 1.5 moles of a gas that is confined in a 3.5 L flask at 760 torr of pressure? R = 0.0821 L atm/mole K (1) 0 °C (2) –245 °C (3) 28 °C (4) 273 °C 24. If the volume of a flask remains constant and the temperature is increased, what happens to the pressure? (1) It increases (2) It decreases (3) It remains the same. CHM1 Review for Exam 10 Part D. Free response. Directions: Provide written responses to the following questions, using complete sentences when appropriate and showing all work for calculations. 25. (6 pts) Dr. VDS has recently synthesized a new uranium compound and needed to determine the molecular weight in order to publish a paper. He determined that 13.4 grams occupied 1.0 L when this compound was in the gas phase at 273 K and 1 atm presure. What was the molecular weight (g/mole) of this compound? PV = nRT, R = 0.0821 L.atm/mole K 26. (5 pts) At STP a balloon has a volume of 4.0 L. What will the volume of the balloon become at 25°C and a pressure of 0.95 atm? 27. (6 pts) Describe three properties of an ideal gas and explain how they would be different for a real gas. CHM1 Review for Exam 10 28. (5 pts) Base your answers to questions 31 and 32 on the following diagram, which shows a piston confining a gas in a cylinder. Using the set of axes provided, sketch the general relationship between the pressure and the volume of an ideal gas at constant temperature. Volume Pressure 29. (5 pts) The gas volume in the cylinder is 6.2 milliliters and its pressure is 1.4 atmospheres. The piston is then pushed in until the gas volume is 3.1 milliliters while the temperature remains constant. Calculate the pressure, in atmospheres, after the change in volume. Show all work. CHM1 Review for Exam 10 Answers 1. 4 2. 1 3. 4 4. 4 5. 3 6. 3 7. 2 8. 1 9. 1 10. 2 11. 4 12. 3 13. 3 14. 1 15. 1 16. 4 17. 1 18. 2 19. 3 20. 2 21. 1 22. 1 23. 2 24. 1 25. 300 g/mole 26. 4.6 L 27. An ideal gas has no molecular volume and as a result is infinitely compressible. Real gases have molecular volumes and become liquids or solids under conditions of high pressure. An ideal gas has no intermolecular forces of attraction and collisions are totally elastic. Real gases have intermolecular forces of attraction (London dispersion forces, dipole-dipole, Van der Waals forces) which cause their collisions to be somewhat inelastic, and causing the real gas to become a liquid or solid at high pressure and low temperatures. 28. 29. 2.8 atm Volume





