Content Help
advertisement

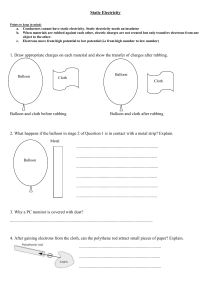
Static Electricity Have you ever run your feet over the carpet in your socks and then touched someone? Ever rubbed a balloon on your hair and then stuck it to the wall? These are common examples of static electricity. Static electricity is a build up of charge on an object. Where does this charge come from? To understand this you need to look at the structure of an atom. An atom is made of a dense center containing protons and neutrons with a positive charge, and electrons with negative charge flying around the outside. The electrons can be removed from the atoms or transferred from one atom to another. Take the example of the balloon and your hair. The balloon collects negative charges (electrons) from your hair. Go to the following web sight to see the charges move. http://phet.colorado.edu/simulations/balloon/webstart.jnlp The balloon gets a build up of negative electrons, and then attracts the positive particles in your hair. Unlike charges attract. Wondering why it is called static electricity? This build up of charge has no where to move, so it’s stationary or static, compared to current electricity which is described as electrons in motion. Static electricity can be discharged though. When you touched someone and got a “spark” it was the discharge of the built up charges. These may seem small, but this is what causes lightning, and what is responsible for the warnings to not get in and out of your car while filling it with gas (the spark ignites the gas fumes). Static electricity is also commercially useful. It is used to paint cars, the paint is charged so it will be attracted to the metal of the car. The same principle explains the new air fresheners that “attract” the pollutant to a metal plate. Smoke stacks use this as well to charge the pollutants and attract them to a surface before they leave the smoke stack. More information: www.sciencemadesimple.com/static.html http://www.mos.org/sln/toe/staticmenu.html Lightning – the big static example: http://weathereye.kgan.com/cadet/lightning/bigspark.html