Static Electricity Physical Science Name: Period: ____ Date: ______
advertisement

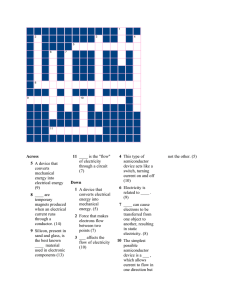
Static Electricity Physical Science Name: ___________________________________________________ Period: ____ Date: ____________ Essential Question: How do I explain the nature of static electricity? Static electricity is a stationary electric charge that is built up on a material. A common example of static electricity is the slight electrical shock that we can get when we touch a doorknob during dry weather. The static electricity is formed when we accumulate extra electrons (negatively-charged particles which we rub off carpeting) and they are discharged onto the doorknob. Producing Static Electricity Everything is made up of atoms, and atoms are made of tiny particles, some of which are electrically charged. Most atoms are electrically neutral; the positive charges (protons in the nucleus or center of the atom) cancel out the negative charges (electrons that surround the nucleus in clouds). Opposite charges attract one another. Similar charges repel one another. Sometimes the outer layer (the negatively-charged electrons) of atoms are rubbed off, producing atoms that have a slight positive charge. The object that did the rubbing will accumulate a slight negative charge as it gets extra electrons. During dry weather, these excess charges do not dissipate very easily, and you get static electricity. (During humid weather, the electrons flow through the damp air and the object become electrically neutral.) Static Electricity Experiments Try this: Rub a balloon on your hair. This removes some of the electrons from your hair and gives the balloon a slight negative charge. Now put the balloon against a wall. It will stick (if the weather is dry) since the negative charges in the balloon will re-orient the atoms of the wall, and a weak electrical force will hold the balloon in place on the wall. Try this (for a really bad hair day): Rub a wool (not acrylic) cap on your hair (on a dry day). This removes some of the electrons from your hair, giving each hair a slight positive electrical charge. Like charges repulse one another, so each hair repulses the other hairs. The result is a mad-scientist hair-do. Lightning In 1751, Benjamin Franklin experimented with electricity in a thunder storm, using a kite, a key and a Leyden jar (two conductors separated by an insulator; it is a device for storing static electricity). The thunder cloud leaked electrons (negatively-charged particles) down through the kite's silk sting to the key and into the Leyden jar (on the ground). Franklin himself was insulated from the electricity; he was holding the portion of the string attached to the string but not directly to the Leyden jar. When Franklin touched the key, he got a static shock. DO NOT TRY THIS - many people have died trying it. In 1752, Franklin developed the lightning rod. Which Objects Lose Electrons Readily? When two materials are rubbed together (like a balloon and your hair), one will lose electrons and one will accumulate them. Physicists have ranked materials by the order in which they lose or gain electrons. This ranking is called the triboelectric series. A small list of some common materials is shown below. When two of these substances are rubbed together, the one that is higher on the list will usually lose electrons (and accumulate a positive charge). The ones on the bottom of the list gain electrons (accumulate a negative charge). http://www.enchantedlearning.com/physics/Staticelectricity.shtml Clarifying questions: 1. What is static electricity? _____________________________________________________________________________________ _____________________________________________________________________________________ 2. How does static electricity form? _____________________________________________________________________________________ _____________________________________________________________________________________ 3. How are most atoms electrically neutral? _____________________________________________________________________________________ _____________________________________________________________________________________ 4. How do charges interact with one another? a. opposite charges ( + ) and ( - ) or ( - ) and ( + ) ___________________________________________________________________________________ b. Similar charges ( - ) and ( - ) or ( + ) and ( + ) ____________________________________________________________________________________ 5. How does a slight positive charged atom form? _____________________________________________________________________________________ _____________________________________________________________________________________ 6. How can object become negatively charged? _____________________________________________________________________________________ _____________________________________________________________________________________ 7. Why do you get more static electricity during dry weather than during humid weather? _____________________________________________________________________________________ _____________________________________________________________________________________ 8. What would happen if you rub a balloon on your hair during a dry day? _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 9. How can you attain a mad-scientist hair-do? _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 10. What was the experiment of Benjamin Franklin? _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 11. Why was Benjamin Franklin insulated in the experiment? Triboelectric Series _____________________________________________________________________________________ human skin _____________________________________________________________________________________ rabbit fur 12. What happened to Benjamin Franklin when he touched the key? glass _____________________________________________________________________________________ human hair _____________________________________________________________________________________ nylon 13. Why should you not do the same experiment? sheep's wool _____________________________________________________________________________________ silk _____________________________________________________________________________________ aluminum 14. Who developed the lightning rod? What is the lightning rod? paper _____________________________________________________________________________________ cotton _____________________________________________________________________________________ wood 15. What happens when two materials are rubbed together? amber _____________________________________________________________________________________ Nickel, Copper, Brass, _____________________________________________________________________________________ Silver, Gold, Platinum ____________________________________________________________________________ acetate, rayon 16. What is the “triboelectric series”? rubber _____________________________________________________________________________________ polyester _____________________________________________________________________________________ PVC (polyvinylchloride plastic) teflon