Bonding Basics Review
advertisement

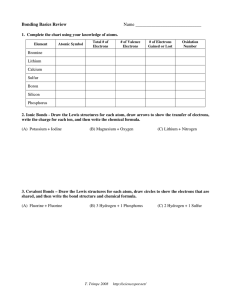
Bonding Basics Review Name _____________________________ 1. Complete the chart using your knowledge of atoms. Element Atomic Symbol Total # of Electrons # of Valence Electrons # of Electrons Gained or Lost Oxidation Number Bromine Lithium Calcium Sulfur Boron Silicon Phosphorus 2. Ionic Bonds - Draw the Lewis structures for each atom, draw arrows to show the transfer of electrons, write the charge for each ion, and then write the chemical formula. (A) Potassium + Iodine (B) Magnesium + Oxygen (C) Lithium + Nitrogen 3. Covalent Bonds – Draw the Lewis structures for each atom, draw circles to show the electrons that are shared, and then write the bond structure and chemical formula. (A) Fluorine + Fluorine (B) 3 Hydrogen + 1 Phosphorus T. Trimpe 2008 http://sciencespot.net/ (C) 2 Hydrogen + 1 Sulfur Bonding Basics Review ANSWER KEY 1. Complete the chart using your knowledge of atoms. Atomic Symbol Total # of Electrons # of Valence Electrons # of Electrons Gained or Lost Oxidation Number Bromine Br 35 7 Gain 1 1- Lithium Li 3 1 Lose 1 1+ Calcium Ca 20 2 Lose 2 2+ Sulfur S 16 6 Gain 2 2- Boron B 5 3 Lose 3 3+ Silicon Si 14 4 Gain/Lose 4 4+ 4- Phosphorus P 15 5 Gain 3 3- Element 2. Ionic Bonds - Draw the Lewis structures for each atom, draw arrows to show the transfer of electrons, write the charge for each ion, and then write the chemical formula. (A) Potassium + Bromine K Br (B) Magnesium + Oxygen Mg (C) Lithium + Nitrogen O Li N K1+ + Br1- KBr Li Mg2+ + O2- MgO Li1+ + Li1+ + Li1+ + N3 Li3N Li 3. Covalent Bonds – Draw the Lewis structures for each atom, draw circles to show the electrons that are shared, and then write the bond structure and chemical formula. (A) Fluorine + Fluorine F F (B) 3 Hydrogen + 1 Phosphorus P H H (C) 2 Hydrogen + 1 Sulfur S H H F F H F2 H P H H T. Trimpe 2008 H H H3P http://sciencespot.net/ S H H H2S