Chapter 7 Covalent Bonds
advertisement
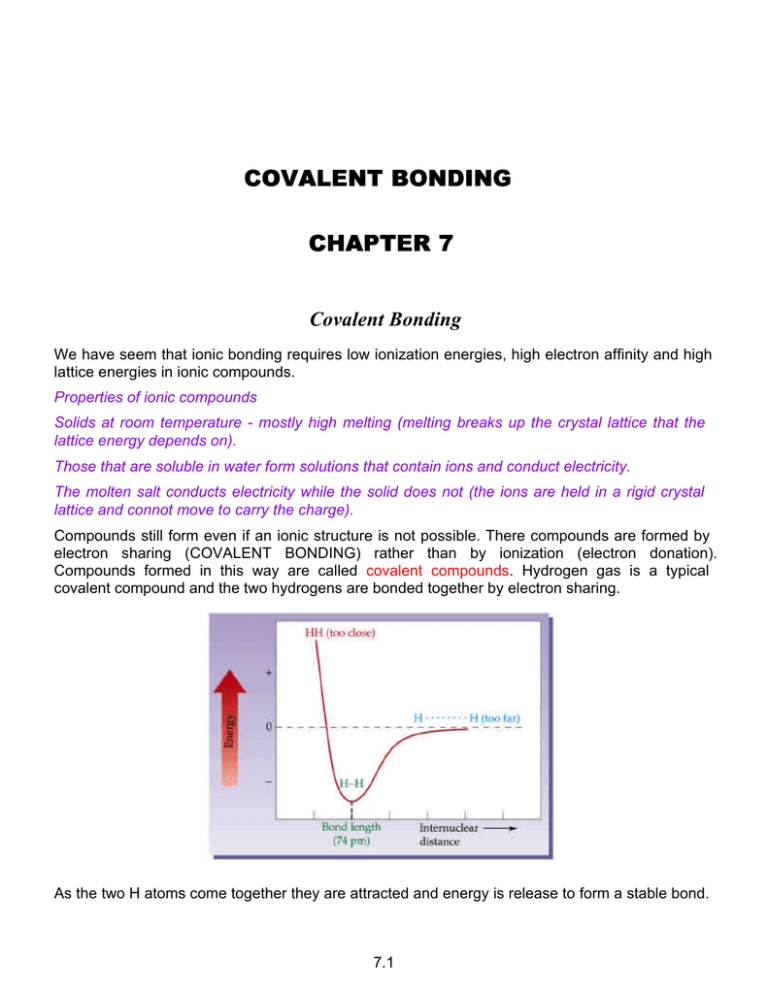
COVALENT BONDING CHAPTER 7 Covalent Bonding We have seem that ionic bonding requires low ionization energies, high electron affinity and high lattice energies in ionic compounds. Properties of ionic compounds Solids at room temperature - mostly high melting (melting breaks up the crystal lattice that the lattice energy depends on). Those that are soluble in water form solutions that contain ions and conduct electricity. The molten salt conducts electricity while the solid does not (the ions are held in a rigid crystal lattice and connot move to carry the charge). Compounds still form even if an ionic structure is not possible. There compounds are formed by electron sharing (COVALENT BONDING) rather than by ionization (electron donation). Compounds formed in this way are called covalent compounds. Hydrogen gas is a typical covalent compound and the two hydrogens are bonded together by electron sharing. As the two H atoms come together they are attracted and energy is release to form a stable bond. 7.1 Whenever a bond is formed energy is evolved and to break a bond there must be an input of energy. Molecular Orbitals (7.13 a little bit to give us a better picture)) We saw that the electron in an atom can be described mathematically using wave theory. In the same way it is possible to do the same thing for electrons in bonds. When the calculations are done to treat the situation of two electrons near two nuclei in a hydrogen atom we find that we get two new orbitals, one of higher and one of lower energy that the hydrogen atomic orbitals. 7.2 Making and energy diagram: The two electrons occupy the lowest energy available molecular orbital - spin pairing into the sigma bonding orbital. if we break the bond energy must be input. The bonds are formed by spin pairing into available orbitals. We will use the simple picture of the electrons spin pairing into the atomic orbitals. N2 N isolated: 1s2 2s22p3 1s2 ↑↓ 2s2 ↑↓ ↑ 2p3 ↑ ↑ The electrons in the currently filling principal energy level are called VALENCE ELECTRONS as it is these electrons that define the chemistry and bonding ability (valency) of the atoms. 1s2 ↑↓ 2s2 ↑↓ 2p3 ↑↓ ↑↓ ↑↓ and this is the configurations of Ne. Again, N in N2: 1s2 2s22p6 the N is not Ne .... it is much more reactive. An early observation was that bonds seem to form between atoms such that the atom attain the same electrons configuration (they become ISOELECTRONIC) with a nearby noble gas. Since this entails 8 electrons (except for He) in the outer shell we have the OCTET LAW. Strength of Covalent Bonds For hydrogen we can measure the strength of the H-H bond: 436 kJ mol–1 + H2(g) → 2 H(g) (This is the reverse of forming the bond). We call this the bond dissociation energy. This is simple enough for diatomic molecules (one bond) but for molecules with more bonds the best that we may be able to do is measure the average bond strength. As well, the bond strength depends on the environment of the bond. It is worth noting that most stable bonds found in nature have energies in the range 350 - 400 kJ mol–1. Weaker bonds lead to a less stable molecule. 7.3 Electronegativity - Polar Covalent Bonds There is not a sudden change from ionic to covalent bonds. Infact pure ionic bonds are probably unknown - perhaps in CsF. In general the bonds that we think of as ionic also have some covalent nature. On the other hand the bonds between the atoms in diatomic pure elements are pure covalent bonds as the electrons are equally shared. 7.4 For the single bond in hydrogen H2 H H in hydrogen fluoride dipole δ+ H δ− F the electrons are ditributed unevenly because fluorine (higher Zeff) attracts electrons more strongly than does hydrogen. The uneven charge distribution leads to the bond electric DIPOLE that is observed and what we call a POLAR BOND. We indicate the direction of the dipole (and sometimes the size) using an arrow (this is a vector). The polarity of bonds tuns out to be a very important bond property. It would be good it there was a simple means of predicting bond polarity based on the relative attraction for electrons for the atom on either end of the bond. Ionization energies and electron affinities are no good as they apply to gaseous atoms and there is no other parameter that can be measured directly that will do the trick. Instead it has been found that exactly how and atom behaves in a bond depnds on many things and we have developed ELECTRONEGATIVITIES that is a composite of some of these. The value varies from 0.7 (Cs) to 4 (F). The greater the difference in the electronegativity between two atoms the more polar (ionic) the bond. Cl2(g) DEN = 0 pure non-polar COVALENT HF(g) DEN = 1.9 58 % ionic polar COVALENT HCl(g) DEN = 0.9 18% ionic polar COVALENT NaCl(s) DEN = 3.1 90% ionic IONIC 7.5 Electron Dot (Lewis) Diagrams. The valence electrons associated with each atom are represented by dots. Increasing across the row until there are eight - an octet. We can use this to easily represent what happens when an ionic compound forms. As we saw earlier, covalent bonds are formed by electron sharing (pairing) into low energy orbitals rather than by donation to form ions. Single bond H2 H H 2 electrons in the bond Multiple bonding Double bond 4 electrons (two pairs in the bond) H2CO H C O H methanal Triple bond 6 electrons (3 pairs in the bond) N2 N N 7.6 Across the Second Period - Stick Bonds We use stick bond to represent two electrons as in the H-F molecule above. A double bond has two sticks and so on. Each bond represents an electron pair. The second row chlorides NaCl BeCl2 BCl3 CCl4 NCl3 Cl2O ClF (on blackboard) Notice that the dot structure for B and Be do not satisfy the octet law but we can draw the dot structure without difficulty Lone Pairs Water .. : O . .H . H . lone pairs (E) O H H (nonbonded or unshared) cf Ammonia NH3 bonded pairs (X) .. H:N:H .. H To simplify diagrams each ”bond” can be replaced by a “stick”. The lone pairs are replaced by 1 rarely! H O N H H H H The molecules here have been shown to have shape .... this is because molecules do have SHAPE! We know that water is bent because it is polar. Similarly ammonia is polar while methan is not. 7.7 The bond angles differ from the tetrahedral angle because the lone pairs repel othe electron pairs more strongly than bonded pairs.Repulsion: LP-LP > LP-BP > BP-BP 7.8 7.9 Hybridization sp3 7.10 sp2 sp Multiple Bonds - Orbital Overlap There are two distinct ways that orbitals can overlap: 7.11 .. :N N : H–N–H H . .. .. .. . . C. 2 .O : ..O :: C ::O .. . .. .. .. O = C = O.. 16 valence ?? O–C–O lone pairs least EN usually "central atom" or 1st in formula 16–4 = 12 left to distribute Make up octets leaves lone pairs Or C 4 O O O2 O2 = 8 = 4 pr 2 pr 2 pr .. .. : .. O :C : : O .. 7.12



