Chemical Bonds
advertisement

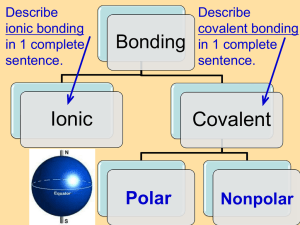
Obj: You will be able to describe the types of chemical bonds & how they form. Do Now: In your own words, define a bond? Please take out pH Lab. HW: Test Review Packet Due Tuesday (11/10) Test Wed. (11/11) Chemical Bonds Chemical Bond • Def: a force that holds together atoms in a molecule or compound – Forms by the losing, gaining, or sharing of electrons! • Form to make atoms stable (outer shell is full) • Types – Ionic – Covalent – Hydrogen Ionic Bonding • Def: Opposite ions are attracted to each other to form a bond Ionic Bonding • Ion = atom with a charge (+ or -) • An example of this type of bonding: salt (NaCl) • This type of boning occurs between metals (left) & nonmetals (right). Covalent Bond • Def: sharing of Electrons to form a bond • Stronger than ionic bonding Covalent Bond • 2 Types: – NONPOLAR equal sharing of electrons • Example – Oil – POLAR unequal sharing of electrons • Example - Water Water • Chemical Formula: H2O • Draw an atom model of water. Like Dissolves Like • Polar molecules and ions all dissolve together • Nonpolar molecules all dissolve together • Polar and nonpolar molecules do not mix together Nonpolar Polar ∂+ - ∂ Covalent bonds Ionic bond + Chemical bonds