Metric Units and Converting
advertisement
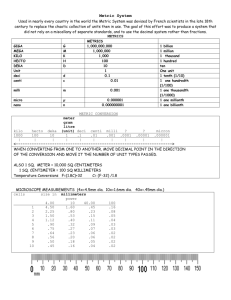
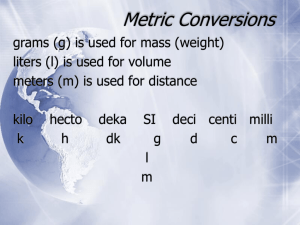
Metric Units and Converting Metric Units • Length – Meters (m) • Mass – Grams (g) • Volume – Liters (L) – Cubic Meters (m3) Kilo – 1,000 Metric Unit Prefixes Hecto – 100 Deka – 10 Base – 1 deci – 0.1 or 1/10 centi – 0.01 or 1/100 milli – 1,000 or 1/1000 Metric Unit Converting • To convert a larger unit (kilo) to a smaller unit (m) you can: – move your decimal to the right. – Mulitiply by the amount off (working with units of 10) • To convert a smaller unit (m) to a larger unit (kilo) you can: – move your decimal to the left. – Divide by the amount off (working with units of 10) Kilo – 1,000 Metric Unit Converting Hecto – 100 Deka – 10 Example converting to a smaller unit: 4,000 m 4 kilo = ________ Base – 1 deci – 0.1 or 1/10 •Move decimal to the right 3 spots since we are 3 tiers off (number gets bigger) •Multiply by 1,000 since we are 1,000 off (4x1,000 = 4,000) centi – 0.01 or 1/100 milli – 1,000 or 1/1000 Kilo – 1,000 Metric Unit Converting Hecto – 100 Deka – 10 Example converting to a smaller unit: 40 4 Deka = ________ m Base – 1 deci – 0.1 or 1/10 •Move decimal to the right 1 spots since we are 1 tiers off (number gets bigger) •Multiply by 10 since we are 10 off (4x10 = 40) centi – 0.01 or 1/100 milli – 1,000 or 1/1000 Kilo – 1,000 Metric Unit Converting Hecto – 100 Deka – 10 Example converting to a smaller unit: 400 cm 4 m = ________ Base – 1 deci – 0.1 or 1/10 •Move decimal to the right 2 spots since we are 2 tiers off (number gets bigger) •Multiply by 100 since we are 100 off (4x100 = 400) centi – 0.01 or 1/100 milli – 1,000 or 1/1000 Kilo – 1,000 Metric Unit Converting Hecto – 100 Deka – 10 Example converting to a larger unit: .020 km 20 m = ________ Base – 1 deci – 0.1 or 1/10 •Move decimal to the left 3 spots since we are 3 tiers off (number gets smaller) •Divide by 1,000 since we are 1,000 off (20/1,000 = .02) centi – 0.01 or 1/100 milli – 1,000 or 1/1000 Kilo – 1,000 Metric Unit Converting Hecto – 100 Deka – 10 Example converting to a larger unit: .002 m 2 mm = ________ Base – 1 deci – 0.1 or 1/10 •Move decimal to the left 3 spots since we are 3 tiers off (number gets smaller) •Divide by 1,000 since we are 1,000 off (2/1,000 = .002) centi – 0.01 or 1/100 milli – 1,000 or 1/1000 Kilo – 1,000 Metric Unit Converting Hecto – 100 Deka – 10 Example converting to a larger unit: Base – 1 .2 Dekameters 200 cm = ____ deci – 0.1 or 1/10 •Move decimal to the left 3 spots since we are 3 tiers off (number gets smaller) •Divide by 1,000 since we are 1,000 off (200/1,000 = .2) centi – 0.01 or 1/100 milli – 1,000 or 1/1000 Now Let’s Practice • 100 mg = ________g • 1 L = ___________mL • 160 cm = ________mm • 14 km = _________m • 109 g = __________kg • 250 m = _________km • 56 cm 6m • 7g 698 mg Now Let’s Practice - ANSWERS 1 • 100 mg = ________g 1000 • 1 L = ___________mL 1600 • 160 cm = ________mm 14,000 • 14 km = _________m .109 • 109 g = __________kg .25 • 250 m = _________km • 56 cm 6m 2 spots to right 6x10 (6m = 600cm) • 7g 698 mg 3 spots to left 698/1000 (698mg = .698g) How to Find Density??? Need to know Mass and Volume First Volume - The amount of space a material takes up. Can be measured 2 ways: • For regular-shaped objects Use length x width x height (L x W X H) Units will be cm3 • For irregular-shaped objects Use water displacement The units will be ml. Volume Practice • Volume of a Regular Shaped Object (LxWxH) – Find the volume of a cube with a side that measures 2.5 cm. (2.5 x 2.5 x 2.5) = 15.63 cm3 – Find the volume of a rectangle with measurements 1cm, 3cm, 4cm. (1 x 3 x 4) = 3 12 cm • Volume of an Irregular Shaped Object (need water displacement) – Find the volume of this pebble. (Read how much the water has been displaced once the pebble was dropped into the graduated cylinder. Had 23 ml to start, then had 30 ml with pebble.) So 30-23= 7 mL 30 mL 23 mL Mass • Mass - The amount of matter in an object. This is measured using a triple beam balance and the units are grams (g) or kilograms (kg). Mass Practice • What are the masses of each rock? 17 g 354.6 g Density • Density - This is a measure of the amount of mass in a certain amount of space or how closely packed the particles of matter are. Density = mass volume M V Units are g/mL or g/cm3 • So you must know, or can be able to figure out mass and volume to find out density. Density Practice • Find the density of an object with a mass of 18 grams and a volume of 2 cm3. (D=M/V), so 18/2 = 9 g/cm3 • Water in a beaker reads 10 mL, add a rock and the water read 24mL. Rock’s mass is 7 grams. What is the density of the rock? (D=M/V, but we need to find our volume 1st). So, 24-10 = 14 mL (this is our volume). Then can do M/V to find density. 7/14 = .5 g/mL Finding Percent Error • Percent Error equation is used to find how far “off” your measurements were from the actual measurements. This is your percent error. Finding Percent Error Practice • What is your percent error if the correct value was 6 g/mL and you calculated 5.4 g/mL? (5.4 g/mL – 6 g/mL) 6 g/mL X 100% = -10% • Errors may include measuring/reading incorrectly, water splashed out of beaker, etc.