Galvanic Cells
advertisement

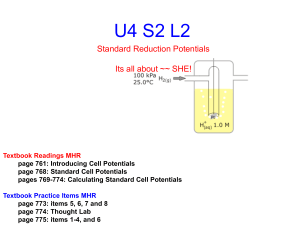
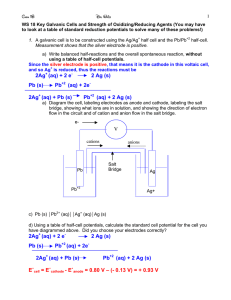
e- e- Electrochemistry Galvanic Cells Chapter 17 Sections 1, 2 &4 ee- e- e- Electrochemistry- Standard Reduction Potentials Activity Series Metals Halogens Li F Rb Cl K Br I Ba Ca Na Mg Al Mn Zn Cr Fe Ni Sn Pb H Cu Hg Ag Pt Au ee- e- e- Electrochemistry- Standard Reduction Potentials ee- e- e- Electrochemistry- Standard Reduction Potentials ep. 795 e- e- e- Electrochemistry – Galvanic Cells Electrons are transferred directly when reactants collide No work is obtained – instead heat is released How can we obtain work? Separate the oxidizing and reducing agents require the e- to go through a wire MnO41- + 8H+ + 5e- Mn2+ + 4H2O Fe2+ Fe3+ + 1e- Oxidation - Fe Reduction - Mn Oxidizing agent - MnO41- Reducing Agent – Fe2+ Current flows for a second then stops Something more needs to added. ep. 792 e- e- e- Electrochemistry – Galvanic Cells It needs a salt bridge Solutions must be connected so that ions can flow and keep the net charge in each container at zero. A salt bridge is a U-tube with electrolyte (pastey stuff) or porous disk. Choose a substance that would be noninteractive (something made of spectator ions if it’s a paste) It allows ions to flow without mixing the solutions ep. 792 e- e- e- Electrochemistry – Galvanic Cells A galvanic cell changes chemical energy to electrical energy. Components 1. Two separate solutions (oxidizing & reducing agent) 2. A wire 3. A salt bridge e- REDUCING AGENT p. 793 ANODE oxidation X Y + e- OXIDIZING AGENT CATHODE reduction X + e- Y ee- e- e- Electrochemistry – Galvanic Cells What is the cathode? Be able to diagram a cell, label the parts and the flow. Which way do e- flow? e- Anode to Cathode Reduction occurs at the cathode. What is happening at the salt bridge? What are the agents? ZnSO4 REDUCING AGENT Copper has a greater reduction potential cations Zn SO4 2- Zn2+ see the atoms p. 793 ANODE oxidation Zn2+ Zn + e- anions CuSO4 Cu Cu2+ OXIDIZING AGENT SO4 2CATHODE reduction Cu + 2e- 2Cu2+ standard reduction potentials ee- e- e- Electrochemistry- Standard Reduction Potentials Line Notation for the cell – so you don’t have to draw the cell • Anode on the left; Cathode on the right • Separate the half cells with a || • Separate the electrode from the solution with a ANODE Zn(s) | Zn CATHODE 2+ (aq) Oxidation chamber electrode | | soln || Cu3+(aq) | Cu(s) || Reduction chamber || soln | electrode ee- e- e- Electrochemistry – Galvanic Cells Cell Potential – the “pull” or driving force that makes electrons go from the reducing agent to the oxidizing agent •The potential to lose eThe push from the element mostly likely to loose to the one that will gain •E cell •Also called electromotive force, emf, of the cell •Unit = volt = 1 joule of work per columb of charge transferred – V=1J/1C •Measured with a voltmeter – Would measure less than cell potential – Because it doesn’t measure the frictional heating of the wire – New voltmeters use a negligible amount of current so they are used •Measured with a potentiometer – Variable voltage device (powered from a cell circuit) – Adjusted so no current flows in the cell circuit e– Then cell potential = voltage setting but opposite sign e- e- e- Electrochemistry – Galvanic Cells Water only spontaneously flows one way in a waterfall. Likewise, electrons only spontaneously flow one way in a redox reaction—from higher to lower potential energy. ee- e- e- Electrochemistry- Standard Reduction Potentials If you don’t want the electrode to participate in the reaction pick a “chemically inert” metal or element. Like Au or Pt. But what would be cheaper? Carbon. Really? Does carbon conduct electricity? Graphite (gr) does! p. 794 ee- e- e- Electrochemistry- Standard Reduction Potentials How would I know how any metal compares against any other? We need a standard – one that can be oxidized or reduced. 2H+ + 2e- H20 reduction H20 2H+ + 2e- oxidation H+ H2+ possible but not common The standard’s E cell would be zero. Standard Hydrogen Electrode or SHE half cell H2 can’t be solid so use a Pt electrode p. 794 ee- e- e- Electrochemistry- Standard Reduction Potentials Zn Zn2+ + 2e- 2H+ + 2e- H2 Can measure total potential of the cell E cell = 0.76 V Can’t measure the potential of the half reactions ( or half cells) Setting the standard potential for the hydrogen half reaction to zero Allows us to assign values to all other half reactions p. 794 ee- e- e- Electrochemistry- Standard Reduction Potentials Denotes “standard state” not the same as STP Standard conditions p. 246 Compounds gases = 1 atm soln = 1M Liquid or solid = pure Element 1 atm 25 C Zn Zn2+ + 2e- E cell = E 2H + H2 2H+ + 2e- H2 + E Zn Zn 0.76 = 0.000V E Zn Zn = 0.76 V 2+ p. 794 2+ + x ee- e- e- Oxidation Reduction Reactions Oxidation States ee- e- e- Electrochemistry- Standard Reduction Potentials ep. 795 e- e- e- Electrochemistry- Standard Reduction Potentials Zn Zn2+ + 2e- Cu2+ + 2e- Cu E cell = E Zn Zn + E Cu Cu 1.10V = 0.76 V + E Cu Cu E Cu Cu = 0.34 V 2+ 2+ 2+ 2+ p. 795 ee- e- e- Electrochemistry- Standard Reduction Potentials The half reaction with the largest potential will run as a reduction (as written) The other will be oxidation; so it will run in reverse So… F2 + 2e- 2F1- E = 2.87 V (reduction) but 2F1- F2 + 2e- E = - 2.87 V (oxidation) E cell = E cathode + E anode reverse the sign of the anode E cell = E cathode - E anode Multiplying the half reaction so that the e- are equal doesn’t change the E E is an intensive property ee- e- e- Electrochemistry- Standard Reduction Potentials About Standard Reduction Potentials •A negative reduction potential means it will most likely oxidize •If the E cell is greater than zero you will get current, spontaneously. •If the E cell is zero then the system has reached equilibrium. The cell is “dead”. •Values in the table are predicting perfect conditions. You probably won’t get this in the lab. Why? – Voltmeter • ee- e- e- Electrochemistry- Standard Reduction Potentials ee- e- e- Electrochemistry- Standard Reduction Potentials This is the chart given with the AP Exam. Half reactions are written as reductions ee- e- e- Electrochemistry- Standard Reduction Potentials The strongest oxidizers (oxidizing agents) have the most positive reduction potentials. The strongest reducers (reducing agents) have the most negative reduction potentials. ee- e- e- Electrochemistry- Standard Reduction Potentials The greater the difference between the two, the greater the voltage of the cell. ee- e- e- Electrochemistry- Standard Reduction Potentials Will Br2 oxidize H2O2? yes Will Cd reduce Ag+? yes Can Al oxidize Au? What are common metals in nature? What are common metal ions in nature? What are common non metal ions in nature? ee- e- e- Electrochemistry- Sample Exercise 17.1 Page 797 a. Consider a galvanic cell based on the reaction Al3+ (aq) + Mg (s) Al (s) + Mg2+(aq) Find the half reactions, balance the cell reaction and calculate E for the cell. ee- e- e- Electrochemistry- Sample Exercise 17.1 Page 797 b. Consider a galvanic cell based on the reaction MnO4 1- (aq) + H+ (aq) + ClO3 1- (aq) ClO4 1- (aq) + Mn2+(aq) + H2O (l) Find the half reactions, balance the cell reaction and calculate E for the cell. ee- e- e- Electrochemistry- Standard Reduction Potentials What if you have a substance that is not solid for the electrode? Look at the example on page 799. It is the same reaction as our Redox Titration Lab Fe2+ + MnO4 1In the example iron is solid but MnO4 1- / Mn2+ is a solution. Fe2+ Fe MnO4 1- Mn2+ Include the inert electrode What is the line notation? Don’t include the water Fe ǀ Fe2+ ǁ H+, MnO4 1-, Mn2+ ǀ Pt ee- e- e- Electrochemistry- Try #29 Page 831 ee- e- e- Electrochemistry- Gold/Nickel Voltaic Cell ee- © 2009, Prentice-Hall, Inc. e- e- Electrochemistry- Standard Reduction Potentials e- ? K+(aq) NO3-(aq) Au3+(aq) Au X-(aq) Ni2+(aq) Ni X (aq) Au3+ + 3e- Au E0 = +1.50 V Ni2+ + 2e- Ni 0 = -0.25 V E A cell will always run spontaneously in the direction that produces a positive cell potential Nickel is the anode E cell = E cathode - E anode flip the nickel Current flows from anode to cathode ee- e- e- Electrochemistry- Standard Reduction Potentials e- K+(aq) Au3+(aq) Au X-(aq) Au3+ + 3e- Au E0 = 1.50 V reduction: cathode NO3 -(aq) e- Ni2+(aq) Ni X (aq) Ni Ni2+ + 2eE0 = 0.25 V oxidation: anode ee- e- e- Electrochemistry- Standard Reduction Potentials Au3+ + 3e- Au Ni Ni2+ + 2e- E0(V) 1.50 0.25 2 Au3+ + 3 Ni 2 Au + 3 Ni2+ 1.75 ee- e- e- Electrochemistry- Standard Reduction Potentials Line Notation: ANODE CATHODE Ni(s)|Ni2+ (aq, 1 M)||Au3+ (aq, 1 M)|Au(s) oxidation reduction ee- e- e- Electrochemistry- Aluminum/Nickel Cell ee- © 2009, Prentice-Hall, Inc. e- e- Electrochemistry- Standard Reduction Potentials e- ? K+(aq) NO3-(aq) Al3+(aq) Al X-(aq) Al3+ + 3e- Al E0 = -1.66 V Ni2+(aq) X-(aq) Ni Ni2+ + 2e- Ni E0 = -0.25 V ee- e- e- Electrochemistry- Standard Reduction Potentials Al Al3+ + 3eNi2+ + 2e- Ni E0(V) 1.66 -0.25 2Al + 3Ni 2+ 3Ni + 2Al3+ 1.41 ee- e- e- Electrochemistry- Standard Reduction Potentials Line Notation: ANODE CATHODE Al(s) | Al3+(aq, 1 M) || Ni2+(aq, 1 M) | Ni (s) oxidation reduction ee- e- e- Electrochemistry- Extra Problem Sketch the Ag/Ag+ & Zn/Zn2+ galvanic cell. Show the direction of e- flow and the direction of ion migration. Calculate E0 for the cell. Give the line notation for the cell. e- ? K+(aq) NO3-(aq) Zn2+(aq) Zn X-(aq) Zn2+ + 2eZn E0 = - 0.76 V Ag+(aq) Ag X (aq) Ag+ + eAg E0 = + 0.80 V ee- e- e- Electrochemistry- Extra Problem Sketch a system having a solid lead electrode and a lead(IV) oxide electrode immersed in sulfuric acid. Show the direction of e- flow and the direction of ion migration. Calculate E0 for the cell. Give the line notation for the cell. e- ? PbSO4 + 2ePb + SO42E0 = - 0.35 V H2SO4(aq) PbO2 Pb PbO2 + 4H+ + SO42- + 2e- PbSO4 + 2H2O E0 = + 1.69V ee- e- e- Electrochemistry- Standard Reduction Potentials The Lead-Acid Car Battery ee- e- e- Electrochemistry- Concentration on E Cu(s) + 2Ce 4+ (aq) 2Cu 2+ (aq) + Ce 3+ (aq) Remember E is positive. Treat this like an equilibrium. Increase [Ce 4+ ], which direction is favored? Forward. Increase the driving force on e-, increase the E Increase [Cu 2+ ] or [Ce 3+], which direction is favored? Reverse. Decrease the driving force on e-, decrease the E Sample Exercise 17.5 page 803 2Al + 3 Mn2+ 2Al3+ + 3 Mn a. [Al3+] = 2.0M; [Mn2+] = 1.0M Al3+ decrease E < 0.48V b. [Al3+] = 1.0M; [Mn2+] = 3.0M Mn2+ increase E > 0.48V ee- e- e- Electrochemistry- Concentration on E Cell Potentials depend on concentration Can construct cells with same components but different concentrations Ag ǀ 0.10M Ag2+ ǁ 1.0 M Ag2+ ǀ Ag E cell =0.80V – 0.80 V = 0 However since the concentration is not equal, the half cell potentials are not 0.80V. This is a concentration cell. Voltages are typically small ee- e- e- Electrochemistry- Concentration on E The Nernst Equation Derived from the dependence of free energy on concentration *** Will learn about this after thermodynamics*** E = E cell - 0.0591 log (Q) n moles of e- Quotient = Keq [P]x [R]y Like Keq but not necessarily at equilibrium Remember (l) and (s) don’t go into the Keq Why not? It’s all about concentration – solids and liquids have a definite volume •The M for solids and liquids is 1 M= mol/L •If you change moles for solids and liquids then the volume changes proportionally ee- e- e- Electrochemistry- Concentration on E The Nernst Equation Derived from the dependence of free energy on concentration *** Will learn about this after thermodynamics*** E = E cell - 0.0591 log (Q) n moles of e- Quotient = Keq [P]x Trying to get the max voltage? [R]y •Choose the half reactions that give max volts – be practical Li or F won’t work •Make the reactant concentration greater than 1 & the product concentration less than 1 That way [P]x / [R]y is 0.XX and then log 0.XX is a negative number and you’re adding to the E cell ee- e- e- Electrochemistry- Sample Exercise 17.7 page 806 Handy ideas about logs log x 2 = 2 log x log 100 = 2 log x y = y log x log 1 x = log 1 – log x = -log x log xy = log x + log y log y√ x = log x1/y = (1/y ) log x Sig. Figs for Logarithms For any log, the number to the left of the decimal point is called the characteristic, and the number to the right of the decimal point is called the mantissa. The characteristic only locates the decimal point of the number, so it is usually not included when determining the number of significant figures. The mantissa has as many significant figures as the number whose log was found. Example 1: log 5.43 x 1010 = 10.735 The number has 3 significant figures, but its log ends up with 5 significant figures, since the mantissa has 3 and the characteristic has 2. ee- e- e- Electrochemistry- Sample Exercise 17.7 page 806 Describe the cell based on the following half reactions: VO2+(aq) + 2H1+ (aq) + e- VO 2+ (aq) + H2O Zn2+ (aq) + 2e- Zn (s) T = 25 C [VO2+] = 2.0 M [H1+] = 0.50M [VO 2+ ] = 1.0 x 10-2 M [Zn2+ ] = 1.0 x 10-1 M (l) ee- e- e- Electrochemistry- Sample Exercise 17.7 page 806 Describe the cell based on the following half reactions: VO2+(aq) + 2H1+ (aq) + e- VO 2+ (aq) + H2O Zn2+ (aq) + 2e- Zn (s) Where T= 25 C [VO2+] = 2.0 M [H1+] = 0.50M [VO 2+ ] = 1.0 x 10-2 M [Zn2+ ] = 1.0 x 10-1 M (l) Solution 1. Balance First 2. Calculate the standard cell potential 3. Use the Nernst Equation ee- e- e- Electrochemistry- Try #59 Page 832 ee- e- e- Electrochemistry- Try #57 Page 832 ee-