Fact sheet
Integrated Supply Management –
optimizing clinical supplies
A single pathway from study planning to closeout
Execute your trials with confidence and efficiency with our integrated supply chain
management solution
Clinical trial designs are becoming more complex, and there are increasing demands on clinical supply
managers to move new therapies through the supply chain more efficiently and cost-effectively. A
seamless and integrated supply chain strategy is critical to helping ensure that the patient receives the
right treatment at the right time.
Quintiles Integrated Supply Management (ISM) is a powerful,
fully integrated solution for biopharmaceutical companies
seeking to increase clinical supply chain efficiencies while
improving the site and patient experience. Powered by
Quintiles, Thermo Fisher Scientific and Cenduit, ISM combines
the clinical operations and supply chain know-how of three
market leading companies to help you:
• Intelligently design your supply chain, specific to the needs
of your individual clinical trial
• Reduce site burden through the use of one central login for
patient and drug management
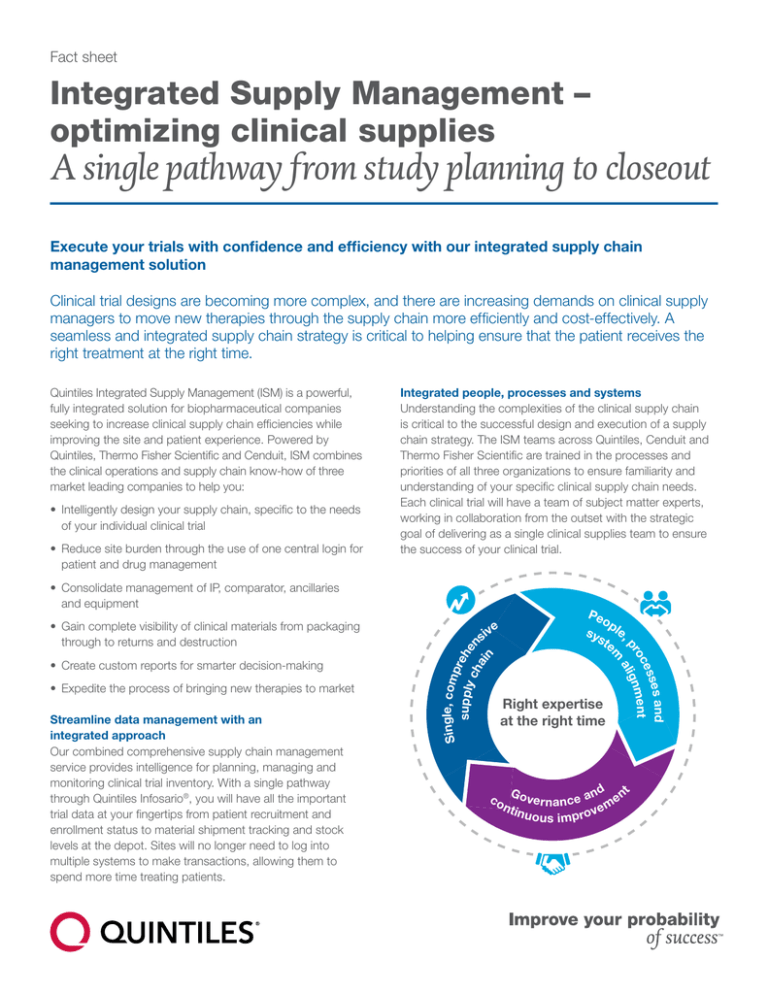
Integrated people, processes and systems
Understanding the complexities of the clinical supply chain
is critical to the successful design and execution of a supply
chain strategy. The ISM teams across Quintiles, Cenduit and
Thermo Fisher Scientific are trained in the processes and
priorities of all three organizations to ensure familiarity and
understanding of your specific clinical supply chain needs.
Each clinical trial will have a team of subject matter experts,
working in collaboration from the outset with the strategic
goal of delivering as a single clinical supplies team to ensure
the success of your clinical trial.
• Consolidate management of IP, comparator, ancillaries
and equipment
• Expedite the process of bringing new therapies to market
Streamline data management with an
integrated approach
Our combined comprehensive supply chain management
service provides intelligence for planning, managing and
monitoring clinical trial inventory. With a single pathway
through Quintiles Infosario®, you will have all the important
trial data at your fingertips from patient recruitment and
enrollment status to material shipment tracking and stock
levels at the depot. Sites will no longer need to log into
multiple systems to make transactions, allowing them to
spend more time treating patients.
Single, comp
r
supply ehe
n
ch
ain
• Create custom reports for smarter decision-making
Peo
p
sys le,
te
ve
si
Right expertise
at the right time
co
es and
ess
oc
ment
pr align
m
• Gain complete visibility of clinical materials from packaging
through to returns and destruction
Go
n d e nt
v
ea
n ti e r n a n c v e m
n u o u s i m p ro
“
“The collective strength of expertise
and capabilities across these three
organizations presents a unique
solution to the biopharmaceutical
development industry.”
– Director Global Supply Chain, Large
Biopharmaceutical Sponsor
Harness the power of intelligent reporting
The seamless integration of clinical trial information among Quintiles, Thermo Fisher
Scientific and Cenduit allows for real-time data sharing. This free exchange of data
empowers our clinical teams to anticipate the needs of the trial, as they are able
to harness that real-time data to forecast material and packaging needs. A robust
reporting tool is fully integrated into Infosario®, placing the most powerful trial
intelligence in the hands of the people who need it most.
Helping to bring new therapies to market faster
Your therapy is important, and we will help you bring it to market faster. By bridging
the gap between clinical operations and clinical supplies, we are tapping into the right
expertise at the right time to help reduce timelines and cut unnecessary costs. Our
integrated approach goes beyond systems integration to include:
• Established governance among all three companies
• Expertise in helping to shape your Clinical Supply Management strategy
and processes
• Planning among all three companies in the early phases of study planning to
ensure that we anticipate your clinical supply chain needs and challenges, so we
can address these well in advance
Contact us
Toll free: 1 866 267 4479
Direct: +1 973 850 7571
Website: www.quintiles.com
Email: clinical@quintiles.com
Copyright © 2016 Quintiles. All rights reserved. 03.0083-1-04.16
A recent simulation exercise
performed on an active study
for a large biopharmaceutical
company indicated that
Quintiles’ ISM strategy has
the potential to reduce
costs and cycle times when
compared with a traditional
supply chain approach.
Gain full visibility on the lifecycle of your new therapy
Your clinical team should feel confident that every patient will receive treatment when
they need it. With Integrated Supply Management, you and your team will be able to
track the whereabouts of clinical materials throughout all stages of the clinical trial. In
addition, your team will have access to an array of self-serve functionalities allowing
them to perform real-time transactions and system updates (e.g. resetting resupply
parameters or adding a new user account). This saves time and helps you avoid
unnecessary trial delays.








