Part 1 Structure and Acid-Base Properties equal to greater than Part
advertisement

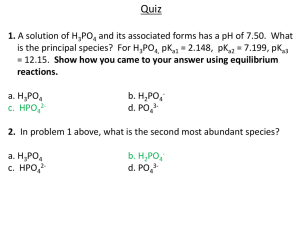
Chem.125/126 Winter 2009 E4 Review Key page1 EXPERIMENT 4 sample Questions Part 1 Structure and Acid-Base Properties 1. A. Based on your knowledge of chemical periodicity, predict the relative acid strength of H2SO4, H3PO4, HClO4, and Mg(OH)2. weakest acid strongest acid Mg(OH)2 < H3PO4 < H2SO4 < HClO4 B. The electronegativity of phosphorous (P) is greater than the electronegativity of arsenic (As). Predict the comparative pH of 0.10M H3PO4 and 0.10M H3AsO4. Indicate (circle) if the pH of 0.10M H3AsO4 is Less than 2. equal to greater than pH H3PO4 (pH 1.5) Below are observed pH readings for a group of acids: A. Based on the pH data, rank the acid strength: 0.10M acid Observed pH Conjugate Base HCN 4.89 CN- HCl 1.00 Cl- HC7H5O2 2.96 C7H5O2 - Acid Strength HCl > HC7H5O2 > HCN Part 2. Conjugate Acid-Base Pairs 2. (continued) B. Complete the above table by filling in the formula for the conjugate base of each acid. C. Based on the above data which 0.10M salt solution has the highest pH: NaCl, NaC7H5O2, or NaCN ? The salt with the highest pH = NaCN 3. Below are observed pH readings for 0.10M solutions of sodium salts: sodium acetate = pH 7.4; sodium benzoate = pH 8.4; sodium lactate = pH 8.0 Predict the comparative acid strength of benzoic, lactic, and acetic acids. Chem. 125/126 Review E 4 W08 Hourly II page 2/3 strongest acid acetic weakest acid > lactic > benzoic Parts 3-4. Acid-Base Neutralization & Indicator Color Changes 4. You perform several titrations (listed below) using 0.01M NaOH. For each titration: − give the formula of the salt that will be formed at neutralization - indicate if the salt and water product is acidic, basic, or neutral − choose the best of four available indicators for each titration. Available Indicators pH range where indicator changes A. Metanil yellow 1.2-2.4 B. Ethyl red 4.0-5.8 C. Litmus 6.0-8.0 D. Thymol blue 8.0-9.6 0.01M Acid Initial acid pH CH3CH2COOH 2.9 HNO3 2.0 5. Salt/acidic, basic, or neutral CH3 CH2 COONa Indicator to use D basic NaNO3 C neutral You titrate 0.193 g of an acid unknown with 0.10 M NaOH and 32.99 mL of base is needed to reach the end point. Your calculated equivalent weight for the acid is 58.50. The titration was performed using phenolpthalein (pH color change interval = 8.2-10.0). You repeat the above titration (i.e. you titrate 0.193g of the unknown acid) but use methyl orange indicator (pH color change interval = 3.2-4.4) . Determine the effect, if any, on: A. The volume of 0.10M NaOH required to reach the end point. B. The calculated equivalent weight of the acid. A. Indicate (Circle) the volume of 0.10 M NaOH used at the end point. more than 32.99mL equal to 32.99mL less than 32.99mL B. Indicate (Circle) the effect of the result on the calculated equivalent weight of the acid more than 58.50 equal to 58.50 less than 58.50 Chem. 125/126 Review E 4 W08 Hourly II page 3/3 Part 5. Identification of an Unknown Acid 6. You are to identify an unknown that is one of the following compounds: Formula MW Mp(oC) Name 17 acetic acid CH3COOH 60 103 3,3-dimethylglutaric HOOCCH2C(CH3)2CH2COOH 160 acid 13 acrylic acid H2C=CHCOOH 72 -23 propanoic acid CH3CH2COOH 74 139-140 maleic acid HOOCCH=CHCOOH 116 31-2 cyclohexane C6H11COOH 128 carboxylic acid -33.8 pentanoic acid CH3CH2CH2CH2COOH 102 186-7 3-chloro, 1,2 benzene ClC6H4 (COOH)2 200 dicarboxylic acid -22.5 4-pentenoic acid CH3=CHCH2CH2COOH 100 You titrate 0.374 g of the unknown compound with 0.100M NaOH. 37.03 mL of base is needed to reach the end point. You now titrate 0.484 g of the unknown compound with 0.100M NaOH and 47.40 mL is required to reach the end point. What is the unknown's equivalent weight? 0.3744g / 0.03703 L x 0.100M = 101.107 eq wt. 0.484g / 0.04740 L x 0.100M = 102.109 eq wt. the equiv. weight = 101.6 Based solely on the calculated equivalent weight, list all compounds that may be the unknown: pentanoic acid,3-chloro, 1,2-benzenedicarboxylic acid , 4-pentenoic acid The unknown compound is a solid at room temperature (25oC). Based on this fact and the calculated equivalent weight, identify the unknown. Based on all the above tests and observations, the unknown most likely is: 3-chloro,1,2-benzene dicarboxylic acid