Alcohol Reactions: Acids, Bases, and Carbocations
advertisement

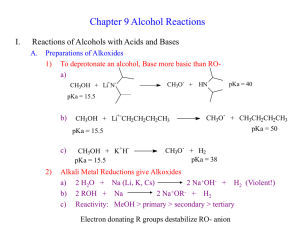
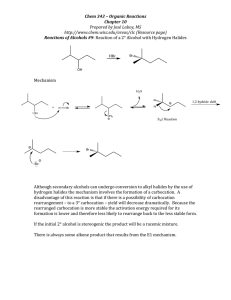
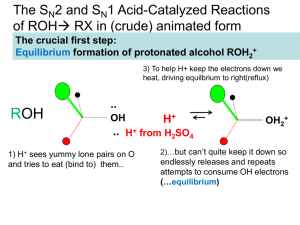
Chapter 9 Alcohol Reactions I. Reactions of Alcohols with Acids and Bases A. Preparations of Alkoxides 1) To deprotonate an alcohol, Base more basic than ROa) CH3OH + Li+N- CH3O - pKa = 40 + HN pKa = 15.5 b) CH3OH + Li+-CH2CH2CH2CH3 CH3O 2) CH3OH + K+HpKa = 15.5 + CH3CH2CH2CH3 pKa = 50 pKa = 15.5 c) - CH3O- + H2 pKa = 38 Alkali Metal Reductions give Alkoxides a) 2 H2O + Na (Li, K, Cs) 2 Na+OH- + H2 (Violent!) b) 2 ROH + Na 2 Na+OR- + H2 c) Reactivity: MeOH > primary > secondary > tertiary 3) B. Uses of Alkoxides a) Strong base for E2 reactions b) Ether synthesis by SN2 reactions (stay tuned!) Protonation of Alcohols form a better Leaving Group 1) X- + ROH RX + OH- (OH- is a poor leaving group) 2) Strong acid converts OH- leaving group to H2O (good leaving group) H + ROH H + R R O + H2O H 3) Synthesis of Haloalkanes from Alcohols ROH HBr H R O Br- RBr + H2O H a) b) Only I- and Br- are nucleophilic enough to work Works best for primary alcohols 4) Reaction of Secondary and Tertiary ROH with H+ a) Secondary and Tertiary alcohols easily lose water to from carbocations b) If the acid contains a good nucleophile, you get SN1 substitution OH c) OH2 Br- SN1 Br If there is no nucleophile or have high Temperature, you get E1 OH d) HBr HBr OH2 -H2O E1 Dehydration = loss of an H2O molecule Use non-nucleophilic acid = H2SO4, H3PO4 OH OH2 H2SO4 o -H2O H Catalytic + + H 130 C e) As usual, Tertiary ROH only does SN1, E1 while Secondary ROH can do SN2, SN1, or E1 in a strong acid II. Carbocation Rearrangement A. Hydride Shift 1) Sometimes we get mixtures of products from 2o and 3o ROH reactions HBr 0 oC 3) + H2O + H normal product H 2) Br H H Br H OH H rearranged product How does the rearrangement occur? a) Carbocation intermediate rearranges b) Hydrogen and both electrons move = hydride shift Mechanism H OH H a) b) + H H OH2 H H:- and + trade places Very fast (faster than SN1/E1) if new C+ more stable -H2O H secondary carbocation tertiary carbon (more stable) - H Br Br H normal product H hydride shift H H Br- Br H H rearranged product 4) Orbital Picture of the Hydride Shift Mechanism 5) 6) Primary ROH/RX won’t form carbocations, so don’t do Hydride Shift Secondary and Tertiary ROH give mixture of products with nucleophile 6) H OH Mixture of E1 Products are also observed at high Temp., no nucleophile H2SO4 H OH2 H H -H2O + o 130 C H H secondary carbocation Me Me H H OH2 H H rearranged products Alkyl Shifts 1) If the carbocation doesn’t have a H- positioned to shift, an alkyl group can move = Alkyl Shift Me OH2 H+ Me H + H Me OH H hydride shift H tertiary carbon (more stable) B. H normal products H H OH2 Me Me H -H2O Me Br- Me Me Br- Me Me Me H Me Me Me H normal product Me H secondary carbocation Alkyl shift tertiary carbon Me (more stable) Me Br Br Me Me Me H Me rearranged product 2) C. Concerted Hydride and Alkyl Shifts in Primary Alcohols 1) Primary alcohol will not form a carbocation, but sometimes rearranged products are observed anyway 2) Only observed with much heat and much time 3) Concerted mechanism explains how Me Me Hydride and Alkyl Shifts occur at about the same rate a) Very fast if rearranged carbocation is more stable b) Formation of tertiary carbocation is faster than secondary Me + H CH2 OH Me CH2 OH2 Me Me Me CH2 Me Br Me III. Ester Formation from Alcohols A. Esters are derivatives of organic and inorganic acids O O 1) R C OH R C OR' Carboxylate Ester Organic Ester O 2) HO P OH O Br - O HO P OR Phosphate Ester Inorganic Ester O Me CH2 Me Me B. C. Synthesis of Organic Esters Subject of chapters 19 and 20 O R C OH O + R'OH R C OR' Synthesis of Haloalkanes from Alcohols involves Inorganic Esters 1) R—OH + HBr R+ multiple products 2) Use Inorganic Reagent to make the water leaving group 3) 1o or 2o ROH + PBr3 1o or 2o RBr + H3PO3 PBr3 3 3 + H3PO3 Et2O Br OH 4) Mechanism RCH2OH + PBr3 -Br SN2 RCH2 O PBr2 RCH2Br + HOPBr2 H 5) 6) Reaction also works to make iodoalkanes with PI3 Chlorination of an alcohol requires thionyl chloride = SOCl2 RCH2OH + SOCl2 NEt3 RCH2Cl + SO2 + HCl 2 more reactions 7) Mechanism RCH2OH + SOCl2 8) D. -Cl RCH2O + SOCl + H - + Cl NEt3 RCH2Cl + SO2 + HNEt3 Amine works as a base to remove H+ from reaction Alkyl Sulfonates in Substitution Reactions 1) Preparation of Alkyl Sulfonates (good leaving groups) O RCH2OH + CH3 S Cl + O Sulfonyl Chloride 2) I- + O N RCH2O S NH CH3 - Cl + O pyridine Substitution reactions take advantage of the good sulfonate leaving group O O O I + -O S CH3 S CH3 SN2 O O O R OH R O S O R' Nu R Nu