Ch 13 Reaction Mechanisms I. Reaction Mechanisms
advertisement
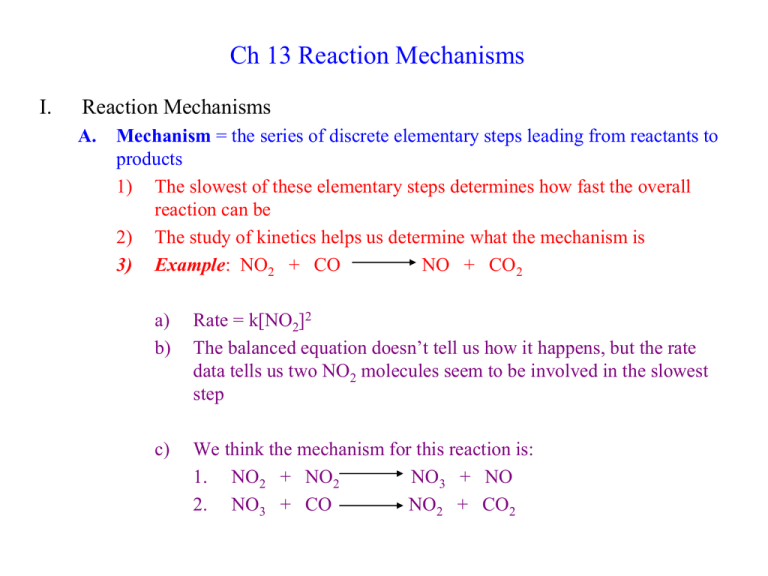
Ch 13 Reaction Mechanisms I. Reaction Mechanisms A. Mechanism = the series of discrete elementary steps leading from reactants to products 1) The slowest of these elementary steps determines how fast the overall reaction can be 2) The study of kinetics helps us determine what the mechanism is 3) Example: NO2 + CO NO + CO2 a) b) Rate = k[NO2]2 The balanced equation doesn’t tell us how it happens, but the rate data tells us two NO2 molecules seem to be involved in the slowest step c) We think the mechanism for this reaction is: 1. NO2 + NO2 NO3 + NO 2. NO3 + CO NO2 + CO2 B. Definitions: 1. Intermediate = molecule produced in one step of a mechanism and used up in another step; it is not isolated as a final product For the following mechanism, NO3 is an intermediate 1. NO2 + NO2 NO3 + NO 2. NO3 + CO NO2 + CO2 2. Elementary Step = reaction whose rate law is written directly from its molecularity a) Molecularity = how many molecules collide in the reaction i. Unimolecular = only one molecule is involved in the step ii. Bimolecular = two molecules collide in the step iii. Termolecular = three molecules collide in the step (this is very rare because of the low probability) 3. b) Since the elementary steps are as simple as possible, we don’t need to analyze experimental data to find their rate laws. We can simply write the rate law directly from the elementary step equation. 1. 2. NO2 + NO2 NO3 + CO NO3 + NO NO2 + CO2 rate = k[NO2]2 rate = k[NO3][CO] Rate Determining Step (rds) = the slowest step in a mechanism a) One step has to be the slowest. b) The overall reaction can’t go faster than that one step, so it determines the rate of the reaction c) The overall reaction rate will be the same as that of the r.d.s d) Example: NO2 + CO NO + CO2 i. Step 1 is the slowest step ii. Overall rate = k[NO2]2 e) Like the narrow point of a funnel, the rds determines the rate C. Mechanisms can’t be proved, only disproved 1) Since we can’t actually see individual molecules react, we can’t prove the exact steps they go through 2) When chemists suggest a mechanism, it must meet two criteria a) The individual steps must add up to the observed reaction equation 1. NO2 + NO2 NO3 + NO 2. NO3 + CO NO2 + CO2 NO2 + CO NO + CO2 3) b) The proposed mechanism must match the observed rate law Observed rate law is rate = k[NO2]2 Step 1 would give this rate law if it is r.d.s. c) If either of these criteria aren’t met, the proposed mechanism is wrong Example: 2 NO2 + F2 2NO2F Step 1 NO2 + F2 NO2F + F (slow) Step 2 F + NO2 NO2F (fast) Observed rate law: rate = k[NO2][F2] II. A Model for Chemical Kinetics A. The Collision Model = Molecules must collide to react 1) Reactions are faster at higher concentrations (rate laws show this; more collisions) 2) Reactions are faster at higher temperatures (molecules are moving faster) B. Activation Energy 1) Chemical reactions happen much slower than predicted based on how many collisions occur 2) Only some of the collisions must lead to reaction 3) Activation Energy = the minimum energy required of a collision to cause reaction a) 2 BrNO 2 NO + Br2 b) The Br—NO bond energy is 243 kJ/mol c) To break that bond, the collision must provide at least that much energy C. Potential Energy Diagrams = plot of energy over the course of a reaction 1. 2. 3. Reactant is on left side and the product is on the right side Activation Energy (Ea) is the amount of energy from the reactant to the highest point (Transition State) Transition State (Activated Complex) = high energy species with a structure intermediate between the reactant and the product. It is never isolated. More Potential Energy Diagrams Rate = k[NO2]2 1. NO2 + NO2 2. NO3 + CO NO2 + CO NO3 + NO NO2 + CO2 NO + CO2 D. Factors Affecting the Reaction Rate 1. Increased Temperature allows more molecules to collide with the minimum amount of energy to react 2. Collision Frequency (z) depends on what kind and how many molecules are reacting 3. Molecular Orientation = how the colliding molecules are oriented during the collision also determines whether or not they will react The Orientation Factor (p) is the fraction of collisions with the correct orientation for reaction. It is always between 01. E. The Arrhenius Equation relates all of the factors to the rate constant k k = rate constant A = frequency factor (combines z and p) Ea = activation energy T = temperature in Kelvins R = gas constant = 8.3145 J/K.mol 1. Ea ln(k) R Taking the natural log of each side gives us another form of the equation that gives a linear plot. lnk vs. 1/T gives straight line with slope = -Ea/R and intercept = ln(A) 1 ln(A) T 2. Example: 2 N2O5 4 NO2 + O2 Ea? T(oC) T(K) 1/T(K) k (s-1) ln(k) 20 293 3.41x10-3 2.0x10-5 -10.82 30 303 3.30x10-3 7.3x10-5 -9.53 40 313 3.19x10-3 2.7x10-4 -8.22 50 323 3.10x10-3 9.1x10-4 -7.00 60 333 3.00x10-3 2.9x10-3 -5.84 3. For only 2 temperatures, the Arrhenius Equation can be rewritten: k2 Ea 1 1 ln k1 R T1 T2 4. Example: CH4 + 2 S2 CS2 + 2 H2S k (L/mol.s) T (oC) T (K) 1.1 = k1 550 823 = T1 6.4 = k2 625 898 = T2 Ea? III. Catalysis A. Catalyst = compound that speeds up a chemical reaction without being consumed itself 1) It is not always possible to speed up a reaction by increasing the concentrations or increasing the temperatures (ex: living things) 2) Catalysts work by lowering the activation energy of a reaction 3) Heterogenous Catalyst: present in a different phase than the reacting molecules. a) This is often a solid not dissolved in the reaction solution b) Reactants Adsorb on the surface to react (a sponge absorbs) c) Example: H2C=CH2 + Pt + H2 CH3CH3 4. Homogeneous Catalyst = Present in the same phase as the reacting molecules. A. Gaseous catalyst in a gas phase reaction B. Ozone Depletion is caused by Cl catalyst from chlorofluorocarbons in the atmosphere O + O3 2 O2 (uncatalyzed = slow) CCl2F2 CClF2 + Cl (source of catalyst) Cl + O3 O + ClO O + O3 ClO + O2 (elem. Step #1) Cl + O2 (elem. Step #2) 2 O2 (catalyzed = fast) B. Enzymes = biological catalysts 1. Most reactions needed by an organism happen too slowly to be of use 2. Enzymes are proteins that have been developed to catalyze almost every biological reaction 3. Examples: Carboxypeptidase A cleavage of other proteins Sucrase cleavage of complex sugars OH CH2 CH - CO2 HO O C CHR H O H C NH2 NH Zn2+ + NH2 NH C O O C O Carboxypeptidase A Sucrase