Law of Definite Composition (4) 2.2 Introduction:
advertisement

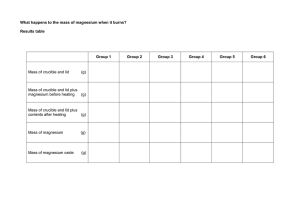
Experiment 2.2 Law of Definite Composition (4) Report=98 Introduction: (5) Elements are a kind of matter that cannot be decomposed by ordinary chemical means. Compounds are chemical combinations of elements. What is a chemical combination as opposed to just any combination of elements? It would help to give an example of what is not a compound. The combination of oxygen and nitrogen in the air is a mixture of the gases. This mixture can be in all proportions of oxygen and nitrogen. The properties of the mixture depend on the properties of the individual components of the mixture. Compounds, on the other hand, are special combinations of the elements. A compound has its own properties, distinct from the properties of its elements. The law of definite composition states that the elements forming a compound always combine in the same proportion by mass. The compound water, H2O, is always a chemical combination of hydrogen and oxygen in a 1:8 ratio by mass. If a mixture of hydrogen and oxygen were reacted, in some other mass ratio, for example 1:2, water would be formed but some hydrogen would remain unreacted. Water forms only in the 1:8 ratio by mass. In this experiment, you will examine the reaction between magnesium metal, Mg, and oxygen O2. When heated, magnesium reacts readily with oxygen in the air. The magnesium will be heated strongly in an open crucible for several minutes. You will measure the mass of the magnesium that reacts and the mass of magnesium oxide that is formed, and use your data to calculate the mass of the oxygen that combined. You can then find the ratio of mass of magnesium to mass of oxygen and compare your experimental ratio with the ratios of your classmates and with the actual ratio. By calculating the percent error you can then evaluate how accurately you performed the experiment and look for sources of error Objectives: (2) 1. Observe a reaction between magnesium and oxygen. 3. Measure masses carefully to obtain accurate results.. 2. Calculate a ratio of mass of magnesium to mass of oxygen.. Materials: (5) Apparatus: laboratory apron sandpaper or emery cloth wire gauze Bunsen burner and lighter ring stand and ring pipestem triangle analytical balance medicine dropper crucible tongs crucible and lid safety glasses •••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••• Reagents: magnesium ribbon deionized water Procedure: 1. Adorn your safety glasses and lab apron. 2. Obtain a piece of magnesium ribbon between 10 cm and 15 cm long from your teacher. If the surface of the magnesium is not shiny, use a piece of steel wool to shine the surface. Set up the ring stand, ring, burner, and clay triangle as show in figure A 3. Obtain a clean, dry crucible and cover, then heat over burner for 4 min. Let cool. Find the mass of the Experiment 2.2 Rev crucible and cover to the nearest 0.0001g and record it on the Report Sheet. 4. Fold the magnesium in an accordion shape, to provide maximum surface area, and place it into the crucible. It is ok if the magnesium brakes, just add it all to the crucible. Find the mass of the crucible, cover, and magnesium. Record it on the Report Sheet. 5. Place the crucible on the clay triangle. Begin heating the crucible gradually with the lid completely on. Heat Page 1 slowly by moving the flame around underneath the crucible. Remove the heat temporarily if a large amount of smoke comes out of the crucible. 7. Turn off the burner and put the lid back on the crucible. Place crucible and cover onto ceramic title. Allow the crucible and cover to cool to a temperature low enough so that you can touch the crucible. Find the mass of the crucible, contents, and cover. Record the mass on the Report Sheet. 8. Add ten drops of deionized water. Smell cautiously, noting any odor. Set up the crucible for heating again. Reheat for four more minutes with the lid on. Let the crucible cool once again. 9. Find the mass of the crucible, cover, and product. Record it on the Report Sheet. Figure A 6. After about 4 min. of direct heating with no smoke, remove the lid slightly. Heat the crucible to redness for 4 min. Finally remove the lid completely and heat strongly for four more minutes. 10. Compare the masses found in steps 7 and 9. If the masses do not agree within 0.005 g, reheat the crucible for 4 min, cool, and find the mass. Repeat until the last two masses agree within this range. 11. Before leaving the laboratory, clean up all materials and wash your hands thoroughly. Data and Observations: (28) (8) Data Table 1 - Primary Data Mass of crucible and cover Mass of crucible, cover, and magnesium Mass of crucible, cover, and product after first heating Mass of crucible, cover, and product after second heating Mass of crucible, cover, and product after third heating (20) Stamp ........................... ••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••••• Calculations (12) - Show all derivations, then record results in Table 2: (2) 1. Find the mass of magnesium that reacted? (2) 2. Find the mass of the magnesium oxide that was produced. (1) 5. According to the periodic table, what is the mass of a magnesium atom? (1) 6. According to the periodic table, what is the mass of an oxygen atom? (2) 3. Find the mass of oxygen that reacted. (2) 4. Find the ratio of the mass of magnesium to the mass of oxygen that reacted to form magnesium oxide, i.e. the experimental value. Experiment 2.2 Rev (2) 7. Calculate your percent error using the formula |Exp Value - Acc Value| * 100 = %error Acc Value Page 2 (5) Data Table 2 - Secondary Data Mass of magnesium oxide produced Mass of magnesium that reacted Mass of oxygen that reacted Exp ratio of mass of magnesium to mass of oxygen reacted % error Analysis and Conclusions: (17) (4) 1. Check with other members of your class to see how your Magnesium/oxygen ratios compare. What is the range of values. Do class results verify the law of definite composition. (4) 2. How would your magnesium/oxygen ratio be affected if not all of the magnesium reacted? Class Data Mg/O Ratio (9) Group Ratio : : : Group Ratio : : : Group Ratio : : : Group Ratio : : : Mean = ___________ Range: ____________ Synthesis: (10) (2) 1. What is the accepted formula for magnesium oxide? (2) 2. Use the accepted value of the Mg/O mass ratio to determine what mass of magnesium would react with 16.0 grams of oxygen. Show derivation. 3. Suppose you tried to react a combination of 42.0 grams of magnesium and 45.0 grams of oxygen. (2) a. Of the two substances, which would not be completely consumed in the reaction? Explain. Experiment 2.2 (2) b. How much magnesium oxide would be formed? (2) 4. When one pound of gasoline, a mixture of hydrocarbons, is burned in an automobile, approximately 3 pounds of carbon dioxide, CO2, is given off. Carbon dioxide is one of the gases contributing to global warming. What information from this experiment helps to explain how one pound of gasoline can give off approximately 3 times as much CO2? Hint: look at periodic table for each element. Page 3