Atoms to Molecules
advertisement

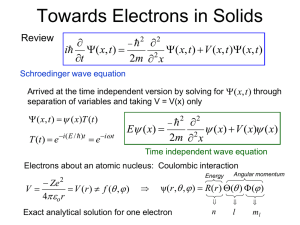
Atoms to Molecules Single electron atom or ion (arbitrary Z) orbitals known exactly Ze 2 V (r ) 4 o r ψ(r , , ) R(r ) ( ) ( ) solutions characterized by QNs: n, l, ml R(rn) 13.6Z 2 En n2 n2 rn Z Consider 1s orbital: 1 0 0 (r, , ) Ae Zr / ao n l ml 1 π ao3 / 2 single electron (neutral) atom is hydrogen, Z = 1 Electrons in Molecules H2+ ionic molecule: 2 protons and 1 electron Take new molecular*symmetry orbital about to bex =a0linear atomic orbitals requirescombination coefficients to be of equal in magnitude Orbitals* 1 0 0 (r, , ) Ae Zr / ao ~ S ψ a ψb ψ ~ A ψ a ψb ψ a b - ½R ½R a b position Probability densities: * = ||2 ||2 Bonding Antibonding electron between nuclei electron on either side of nuclei a b - ½R ½R a position intuitively: this orbital has lower coulombic energy b “node” high energy Types of MOs from LCAOs s s + pz pz + s bonds s pz + px (py) + bond Types of MOs from LCAOs s s + Showed: symmetric = bonding s bonds Symmetric pz + Anti-bonding pz + + Antisymmetric + + + + Bonding + + + + The H2 Ion Vary inter-nucleus distance, R, between the two protons R0 anti-bonding E b a R ~ A ψ a ψb ψ electron sees only one proton, no interaction -13.6 eV bonding E 0 R0 R R a b To find equilibrium bond distance, R0 ~ S ψ a ψb ψ The H2 Molecule • Introduce 2nd electron • Solution has perturbations due to electron-electron interactions • Ignore these and place 2nd electron in ‘same’ bonding orbital but with opposite spin alternative depictions 1s R E H H 2 anti-bonding states -13.6 eV 2 protons 2 bonding states • Result: H2 covalent bond • Directional; typical of molecules two 1s states each 4 states total 1s The N-atom Hydrogen Solid 1s1 N-atom solid N electrons Chemical Bonding Continuous Bands R0 E N states unoccupied R overlap of states discrete continuous N anti-bonding states 1s N states occupied N bonding states 2N states H2 molecule: N = 2 1s orbital is close to nucleus, nuclear interaction prevents strong overlap Lithium: A Simple Metal Li #3 1s22s1 N-atom solid 2s orbitals overlap without nuclear repulsion N 2s electrons, 2N states R0 R E N states unoccupied N states occupied anti-bonding overlap of states discrete continuous 2s 2N states 1s 2N states bonding E 0 R0 R All states occupied, independent Silicon: A Semiconductor N-atom solid 4N relevant electrons Si: #14 1s22s22p6 3s23p2 [3(sp3)4] hybrid orbital composed 3s: 2N states [Ne] of 3s and all 3p orbitals: Hybridization: consider just 2 atoms R0 anti-bonding 3 3p 3s 1 6 states 2 states 3p 3s 4N states unoccupied Eg 1 3 bonding 4 states (+ 4) N-atom Solid Continuous Bands 8N states R overlap of states discrete continuous E 4 states (+ 4) 3p: 6N states 4N states occupied E 0 R0 R 4N anti-bonding states “sp3” 8N states 4N bonding states Magnesium: A Metal? Mg: #12 1s22s22p6 3s2 N atom solid, 2N electrons [Ne] R0 R 3s and 3p overlap to create a band with 8N states; only 2N states occupied yes, a metal E 3p 2N states occupied 6N states anti-bonding 3s 2N states bonding E 0 R0 R metal requires a partially occupied band?? LiF: An Ionic Solid Li: #3 1s22s1 F: #9 1s22s22p5 1s2 2p6 Li+1 + e F + e- F-1 Energy of bonding for a hypothetical ion pair E = Eionization + Ecoulombic Eion ( Li) Eion ( F ) 13.6 Li Z eff 2 13.6 Eion 13.6Z 2 n2 2 5.4 eV > 0 requires energy -3.7 eV < 0 releases energy -7.2 eV 2 F Z eff 22 2 +1 -1 ECoul Z1Z 2 e 2 4 o R Zeff < Zact due to shielding Epair = 5.4 - 3.7 - 7.2 eV = 5.5 eV N-atom Pair Solid of LiF Li: #3 1s22s1 F: #9 1s22s22p5 e: Li(2s) F(2p) Li+1 + e- 1s2 2p6 E(F2p) < E(Li2s) 6N electrons R0 R LiF is a non-metal E Li 2s 2N states Eg 6N states occupied E 0 R0 R F 2p 6N states Thoughts on how to transform it a metal? Summary: MO/LCAO Approach • N atom solid – bonding and anti-bonding states – isolated states bands due to exclusion principle • Metal – no energy gap between occupied and unoccupied states – many need to consider orbitals of slightly higher energy • Semi-conductor – hybrid orbitals bands – ‘small’ bandgap between occupied and unoccupied • Ionic qualitative distinction – electron transfer from electropositive to electronegative ion – orbitals bands – ‘large’ bandgap between occupied and unoccupied states