Photoelectric Effect Lab Introduction: Three fundamental questions of physics are:
advertisement

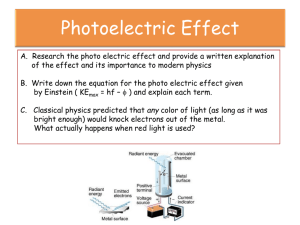
Photoelectric Effect Lab Introduction: Three fundamental questions of physics are: 1) What is matter? 2) What is energy? 3) What is the nature of the interaction between energy and matter? These questions are every bit as important today as they were at the turn of the 1900’s. In order to shed light upon the answers to these questions, Physicists have performed numerous experiments over the past 100 years. The results of these experiments have caused us to change our view of the world and form the basis for many subfields of engineering and science. In particular, the study of light energy has led to compact disks, laser surgery, optical detectors and communication, laser chemistry, checkout registers at Wal-Mart and many other important technologies. The high speed of light has also challenged our very notion of space and time. Although the prevailing theories appear to predict the answer to every experiment that has been tried, history tells us that the correct answer to these questions is probably still not known. Future experiments will probably demonstrate that our present theories are only approximations of the true answers to nature’s questions. One of the most important experiments from the early 20th century was the photoelectric effect experiment. In this experiment, shinning a light upon a metal surface may cause electrons to be emitted from the metal. It is hard to believe that such a conceptually simple experiment could change the course of physics or lead to a Nobel Prize. However, Albert Einstein was awarded the Nobel Prize in Physics for his correct prediction of the results of this experiment in 1905 using a new model for the nature of light. Robert Millikan, co-founder of the California Institute of Technology and fellow Nobel Prize Winner, performed the careful experimental verification of Einstein’s predictions. Basic Experimental Concepts: An electron in a metal can be modeled as a being in an average potential energy well due to the net attraction and repulsion of protons and electrons. The minimum depth that an electron is located in the energy well is called the work function of the metal,. Since different metal atoms have different number of protons, it is reasonable to assume that the work function depends on the metal. This is also supported by the fact that different metals have different values for electrical properties that should depend on the electron binding including conductivity. The electron in the potential energy well of a metal is shown below. It is analogous to a marble trapped in a water-well. The shallower the well (i.e. the lower the work function) then the less energy required to cause the emission of E=0 E = -e the electron. Electron filaments are often coated with Cs to reduce the work function in TV’s and other electron emission devices. If we shine a light with sufficient energy then an electron is emitted. By conservation of energy, the kinetic energy of the emitted electron, T, is related to the energy of the light absorbed, E, and its initial potential energy in well, U, by the formula TEU For a fixed light energy, an ejected electron will have the maximum kinetic when it is initially at the minimum depth in the potential energy well. Thus, we have Tmax E ( e Φ) E e Φ An emitted electron’s maximum kinetic energy can be determined by using an electric field that attracts the electrons back to the metal. Consider a small metal collector surrounded by a metal electron emitter as shown below. If the emitter is floated (not in electrical contact with another conductor) then it will become positively charged as electrons leave the metal. The electrons striking the collector will likewise charge the collector negatively. The collector-emitter plates form a capacitor that is charged when electrons move across the gap. Eventually, the electric field prevents even the most energetic electrons from crossing the gap. Thus, the capacitor is fully charged. Light Emitter Collector The voltage on the fully charge capacitor is called the stopping potential Vstop. Using our physics knowledge from PHYS2424 and conservation of energy, we can now determine the maximum kinetic energy of an electron by Tmax e Vstop Plugging this result into our previous work, we find that e Vstop E e Φ Dividing by the magnitude of the electrons charge, e, we have that Vstop E Φ e You will be measuring the capacitor’s stopping potential for different combinations of light intensity and color combinations. Because a capacitor’s voltage changes when charge is removed or added, you will be connecting your voltmeter to the capacitor through a high impedance FET amplifier to minimize charge transfer. Before each measurement, you will need to discharge the capacitor. This is performed by pushing the “zero button” which momentarily connects a low resistance path between the capacitor plates. Note: I said that the high impedance FET minimized charge transfer. I didn’t say that it totally eliminated it. The light source for the PASCO h/e experiment is a mercury vapor light with an optical grating. The grating spreads the white color light emitted by the mercury vapor light into a repeating spectrum of its component colors. The special fluorescent screen on the PASCO h/e detector fluoresce a bluish color when irradiated by ultra-violet light. This will enable you to align the normally invisible ultraviolet light with the detector. The figure below shows the composite light along with the 1st order diffraction spectrum will look on the fluorescent screen. The wavelengths for these colors are provided in the table. White UV Violet UV 365.483 nm Violet 404.656 nm Blue 435.835 nm Green 546.074 nm Yellow 578 nm If you adjust the detector arm, you will observe that the diffraction patter is repeated. These are the higher order diffraction spectra. You will also note that the spacing between the colors gets less as you move to higher order diffraction spectra. You may use these spectra in your investigation provided you can isolate a particular color line. In the 1st order, you may also note a continuous red section of the spectrum and other less intense continuous color sections. To isolate any color dependent effects in your investigation, it is important that only one color of light strike the detector during each measurement. To isolate green and yellow lines from near-by colors and light contributions from higher order diffraction spectra, you need the attach the appropriate green or yellow filter in front of the detector for these measurements. You will also make measurements as a function of the light intensity. This is accomplished by attaching a transmission filter in front of the detector to block some of the light. Investigation: I. Questions 1. Does the color of light affect the maximum energy of the electrons emitted from the metal? 2. Does the color of light affect the rate at which the electrons are emitted from the metal? 3. Does the intensity of the light affect the maximum energy of the electrons emitted from the metal? 4. Does the intensity of the light affect the rate at which the electrons are emitted from the metal? 5. For different intensities of light is the start of electron emission instantaneous with the application of light or does it take a considerable amount of time? 6. Provided you wait a sufficient amount of time, will an electron always be emitted when you shine light on a metal? II. Pre-Lab Work Prior to performing the lab, you and a partner are to determine what both the classical theory and Einstein’s corpuscular theory of light predict as answers for these questions. There are several modern books that cover the photoelectric effect in some detail. A good initial reading source is Quantum Physics by Eisberg and Resnick. You also need to read your oral and written report requirements. III. Lab Work During lab, you will be provided time to make measurements to check the predictions of both theories. You must decide the type and number of measurements that you need to make in order to answer the questions above and to fulfill the oral and written requirements listed below. This is an upper level class for students getting ready to become working scientists and engineers. I will answer specific technical questions but I will not give you a cook book measurement procedure to use. If you don’t know what to measure, then you probably haven’t successfully completed your pre-lab work. IV. Oral Report You are required to present your research during a 20 minute scientific talk in the format of an American Physical Society presentation. You will be given 17 minutes to present your results followed by 3 minutes of questioning by your peers and faculty. I will grade both your presentation and your questioning of other groups. You may use either overheads or Power Point to make your presentation. The talk must cover the following basic sections: abstract, theory, experimental procedure, results, and conclusion. In the results section, you must present a data table with stopping potential versus frequency and a data table with stopping potential versus intensity for each color. Each data entry must include the data’s associated uncertainty. The procedure that you used to compute or obtain this uncertainty must also be discussed. Your talk must also include a graph of the stopping potential versus frequency and a graph showing the time required to reach 93% of the stopping potential for ultra-violet light versus light intensity. You are to make conclusive arguments using your experimental data as to the correctness of each theory in answering each of the six questions above. You are also to provide your experimental values with uncertainties for the work function of the metal used as the emitter and for Planck’s constant. Your group will need to practice your talk otherwise you will either be disorganized or run out of time. V. Written Material Following your talk, you are to hand me a hard copy of your graphs and data tables along with your uncertainty calculations. I may ask for additional material related to questions that I have based upon your talk or written material.