Water Most important and abundant biological molecule
advertisement
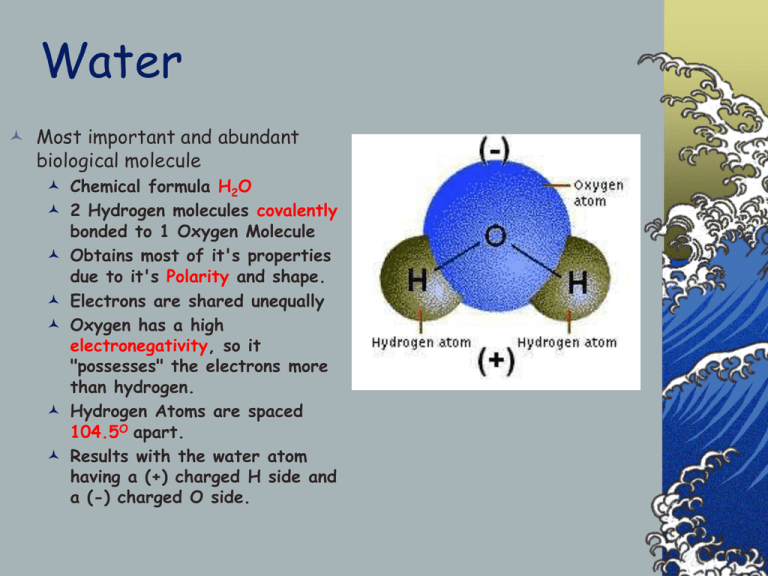
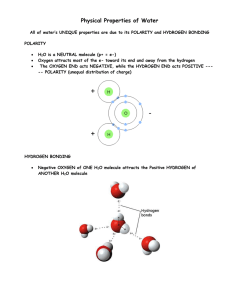
Water Most important and abundant biological molecule Chemical formula H2O 2 Hydrogen molecules covalently bonded to 1 Oxygen Molecule Obtains most of it's properties due to it's Polarity and shape. Electrons are shared unequally Oxygen has a high electronegativity, so it "possesses" the electrons more than hydrogen. Hydrogen Atoms are spaced 104.5O apart. Results with the water atom having a (+) charged H side and a (-) charged O side. Hydrogen Bonding High polarity results in H2O's ability to form Hydrogen Bonds. Hydrogen bonds are formed by the attraction of the charged portions of the H2O molecule to the charged portion of other H2O molecules, Ions, or other polar molecules. Cohesion This allows for high cohesion properties of water (ability to attract to itself). Allows water to have high specific heat, and heat of vaporization because of the strong attraction of H2O molecules. This also explains why water actually expands as it freezes (Note: There is something SERIOUSLY wrong with this diagram. What is it?) Adhesion Adhesive properties make H2O a powerful solvent. Polar portions of H2O molecule can "pluck off" Ions from an Ionic crystal lattice. Interactions with Non-polar Molecules Water Organizes nonpolar molecules. Nonpolar molecules are hydrophobic (repelled by H2O) Repulsion forces these molecules into particular arrangements. Example is the lipid bilayer found in cellular membranes. Water and pH Water disassociates (ionizes) in itself. Basis for the pH scale of acid - base measurement. Water can disassociate from H2O to H+ (Hydrogen Ion, acid) and OH- (Hydroxide Ion, base). Usually does this for 1/10,000,000 or 1E-7. pH is a measure of this dissociation. According to the following formula: pH = -Log [H+] Lower pH indicates high H+ concentration - high acidity. Higher pH indicates low H+ concentration (high OHconcentration) - High alkalinity. A neutral solution has pH=7.