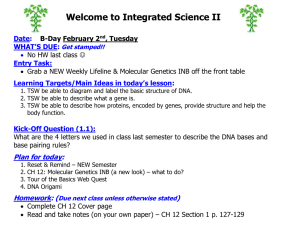
Welcome to Chemistry Date: Entry Task:
advertisement
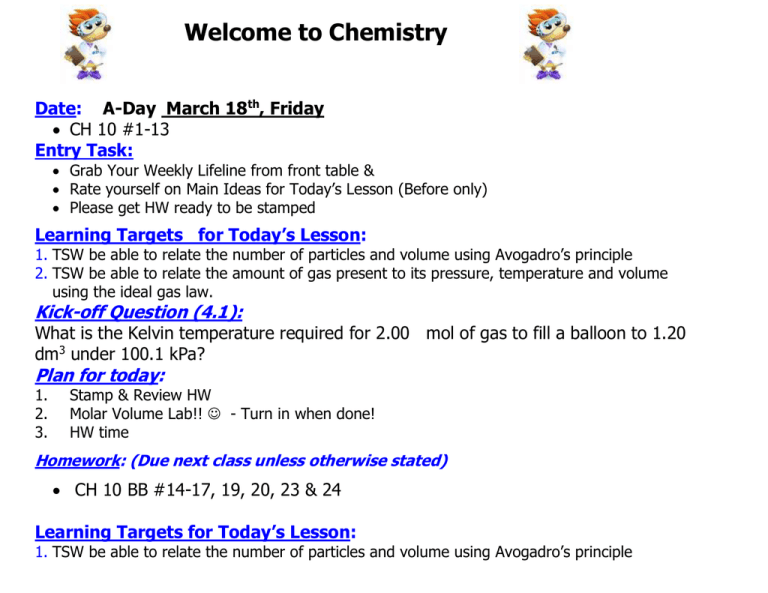
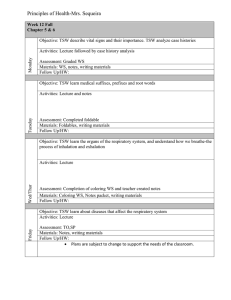
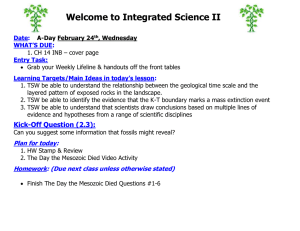
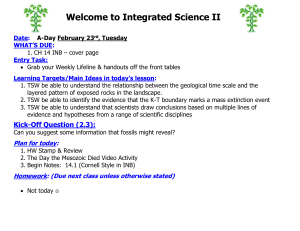
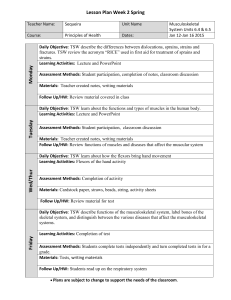
Welcome to Chemistry Date: A-Day March 18th, Friday CH 10 #1-13 Entry Task: Grab Your Weekly Lifeline from front table & Rate yourself on Main Ideas for Today’s Lesson (Before only) Please get HW ready to be stamped Learning Targets for Today’s Lesson: 1. TSW be able to relate the number of particles and volume using Avogadro’s principle 2. TSW be able to relate the amount of gas present to its pressure, temperature and volume using the ideal gas law. Kick-off Question (4.1): What is the Kelvin temperature required for 2.00 mol of gas to fill a balloon to 1.20 dm3 under 100.1 kPa? Plan for today: 1. 2. 3. Stamp & Review HW Molar Volume Lab!! - Turn in when done! HW time Homework: (Due next class unless otherwise stated) CH 10 BB #14-17, 19, 20, 23 & 24 Learning Targets for Today’s Lesson: 1. TSW be able to relate the number of particles and volume using Avogadro’s principle 2. TSW be able to relate the amount of gas present to its pressure, temperature and volume using the ideal gas law. Exit Question (4.2): NONE TODAY