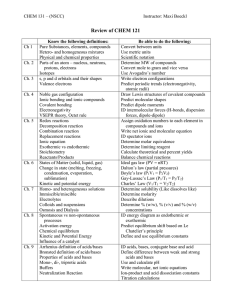
Chapter 11 Water and Solutions
advertisement

Chapter 11 Water and Solutions Water • The universal solvent. It has the ability to dissolve many molecules. • In living systems these molecules can then be transported from one place to another by diffusion or by some kind of a circulatory system. • Liquid water has a higher density than ice (solid water). Ice thus floats on the surface of liquid water. Fish and other organisms can then live below the ice in natural bodies of water. • Water has a very high specific heat. Therefore, large bodies of water can moderate temperature by absorbing great amounts of heat. • Water has a very high latent heat. This means that a great amount of heat is needed to evaporate water. This is what occurs when people perspire, we get rid of a lot of heat from our bodies, thus enabling us to withstand high temperatures. Bonds in the Water Molecule • The H-O bonds in the water molecule are polar covalent. The oxygen atom attracts the electrons more than the hydrogen atoms because it is more electronegative. • Since the molecule has a “V” shape: O Negative end H H Positive end • The oxygen end of the molecule is therefore more negative and the hydrogen end of the molecule is more positive. • The molecule is said to be polar and thus possesses a dipole (a negative and a positive end). Hydrogen Bonding • Due to the dipole moment in the water molecule water has many unusual characteristics. • Hydrogen bonding occurs whenever a H atom is bonded to an O, N, or F atom, since these are the most electronegative elements. The electrons are pulled very close to the O, N, or F atom and therefore the O, N, or F atom becomes partially negative whereas the H atom bonded to it becomes partially positive. • A dotted line is used to represent a hydrogen bond, as opposed to the solid line which is used for a single bond. Hydrogen Bonding • The partially negative oxygen of one water molecule attracts and is hydrogen bonded to a partially positive hydrogen of another water molecule. This happens throughout the water molecules present in a sample of water. Effects of Hydrogen Bonding • Due to the hydrogen bonding in the water molecules water exhibits all the unusual properties that it is known for and which ultimately permit life on earth to exist. • Due to the great attraction of water molecules for one another the boiling point and melting points of water are very high for such a small molecule, the molecules like to stay as close to one another as possible. • Other small molecules like water tend to be gases at room temperature and do not become solids unless the temperature is extremely low, much lower than 0oC. Hydrogen Bonding in Ice Hydrogen bonding occurs in solid water (ice), but it occurs as part of a very organized crystalline structure in the forms of hexagons. When the ice melts the molecule collapses from its highly organized crystal structure to a more compact structure which permits greater approach of the hydrogen bonded H and O atoms. This is why liquid water is more dense than ice. Dissolving Process • There are two types of mixtures: • Heterogeneous mixtures, like oil and vinegar, or sand and water, are those where two or more distinct layers exist. The two substances are not soluble in one another. • Homogeneous mixtures, like table salt in water or sugar in water, are those where there is only one layer, since the substances are soluble in one another. A homogeneous mixture is a solution. • A homogeneous mixture that is in the liquid state can be recognized because it is clear (transparent). It can be colored or colorless. Solutions • A solution is made up of a solute and a solvent. • The solvent is the substance which is present in the higher amount. The solute is present in the lesser amount. • Solutions can be in the gas, liquid or solid states, although the most common are in the liquid state. • Solutions in the liquid state can have a gas, a liquid, or a solid dissolved in a liquid. • Air is a gaseous solution made up of 78% N2, 21% O2, .9% Ar, and other miscellaneous gases. Therefore N2 is the solvent. Solutions • Aqueous solutions are those in which the solvent is water. Aqueous comes from aqua, the latin word for water. The solute can be a solid, a liquid, or a gas. • A solution becomes saturated when the maximum amount of solute is dissolved in the solvent. • A solution which has less than the maximum amount of solute dissolved is unsaturated. • If more than the maximum amount of solute is added to a solvent than can dissolve in it at a certain temperature, then the excess will settle on the bottom of the container (in the case of a solid solute) and the solution itself will be saturated, since only the maximum that can dissolve will be dissolved. • For example, if the maximum solubility of substance “A” in water is 20 grams of A per 100 grams of water: Adding 15 grams of A to 100 grams of water-Unsaturated Adding 20 grams of A to 100 grams of water-Saturated Adding 30 grams of A to 100 grams of water-Saturated Adding 20 grams of A to 150 grams of water-Unsaturated Adding 10 grams of A to 50 grams of water-Saturated Degrees of solubility • Miscible substances: When there is not limit to how much of a solute can dissolve in a solvent. • Mixtures of gases are always miscible. • Like dissolves like: Two polar substances tend to dissolve in one another. Two non polar substances tend to dissolve in one another. Water and ethyl alcohol are both polar and are miscible in one another. Degrees of Solubility • Water is a polar substance, CCl4 (carbon tetrachloride) is a non-polar substance, since even though the C-Cl bonds are polar the molecule has a symmetrical geometry, so it does not have a more positive or a more negative end. • CCl4 will dissolve greases and oils, which are non polar. • Soap consists of a molecule which has a part that is polar and a part that is non polar. Oils and grease will not dissolve in water alone. However, soap can be dissolved in water through its polar end and it will in turn dissolve oils and grease and is therefore used for cleaning. Geometry of CCl4 Cl Cl C Cl Cl The less electronegative atom, the C, is in the middle symmetrically surrounded by Cl atoms in the form of a tetrahedron. Therefore the molecule does not have a more positive or more negative end and it is nonpolar. Dissolving Ionic Compounds in Water • Many ionic compounds, like NaCl, are soluble in water because the Na+ is attracted to the partially negative O of the water molecule. The Cl- is attracted to the partially positive H of the water molecule. • This is called hydration, since the ions become surrounded by water molecule. Dissolving Ionic Compounds in Water • There is also the effect of the great attraction that positive and negative ions in an ionic compound have for one another. • For an ionic compound to be soluble in water the attraction of the water molecules and the ions has to be greater than that of the ions for one another. • Saturation occurs because there are less water molecules available to compete for the ions. It is like a tug of war. • Ionic compounds that have a greater attraction for its ions than for water are said to be “insoluble” in water. Usually some miniscule amount will dissolve when the compound is considered insoluble. Often this will be ionic compounds which have ions that have +2 and -2 charges or higher. Most ionic compounds that contain at least one ion with a +1 or -1 charge are soluble in water. Ionic Compounds dissolved in Water • NaCl(s) CaCO3(s) H2O H2O Na+(aq) + Cl-(aq) CaCO3(s) Concentration of Solutions • Concentration refers to the amount of solute which is dissolved in a given amount of solvent. • In a qualitative manner when the concentration is high the solution is said to be concentrated. When the concentration is low the solution is said to be dilute. Concentration of Solutions • In a quantitative manner one can express concentration of solutions in many ways. One of the ways that are used are: • % by volume= volume of solute x 100 volume of solution • An example is rubbing alcohol, which can be, for example, 12% by volume. This means that there are 12 mLof isopropyl alcohol per 100 mL of aqueous solution. Concentration of Solutions • % by mass=mass of solute x 100 mass of solution • The mass can be in grams or any other mass unit in the metric system. It could also be in weight units in the English system, like ounces. % by weight is equivalent to % by mass. • Hydrogen Peroxide, H2O2 is sold as 3% by weight, which means that there are 3 ounces of H2O2 per 100 ounces of aqueous solution. 2 parts solute x 100 = 2% 100 parts soln 2 mL solute 100 mL soln 2 g solute 100 g soln x 100 = 2% x 100 = 2% The % by mass of salts in salt water (salinity) is: 35 g salts x 100 = 3.5 % 1000 g soln Solubility of Ionic Compounds • Solubility is defined as the maximum amount of a solute that will dissolve in a given solvent at a specified temperature. When you reach this maximum amount of solute the solution is said to be saturated. • For example, adding sugar to tea. If you add too much sugar, some will not dissolve because the solution becomes saturated. • For most solutions solubility increases with temperature. There are exceptions, however. Solubility of Gases • Gases are the opposite of the majority of ionic compounds when it comes to the relationship between solubility and temperature. • The higher the temperature the lower the solubility of gases in a solution. • If you open up a soda can at room temperature more of the dissolved carbon dioxide gas will escape and the soda will become flat faster than if you open it after just taking it out of the refrigerator. This is because the carbon dioxide gas is less soluble in water at the higher temperature. Electrolytes • Electrolytes are substances or solutions which conduct electricity. Often these will be aqueous solutions. • In order to conduct electricity there has to be a flow of electrons through the solution. • In order for there to be a flow of electrons through a solution there have to be ions in the solution. • The more ions there are the stronger the electrolyte will be. Only ionic compounds Or acids dissolved in water will produce ions in solution and electricity can be then conducted through the Solution. Non Electrolytes • All covalent compounds except for acids are non electrolytes, since no ions are present in solution. • Water is a covalent compound, so it is a non electrolyte, so are all alcohols and most other substances which are liquids or gases at room temperature. • Acids are unique, since they are covalent compounds but they ionize when dissolved in water. All acids are soluble in water. Aqueous Solutions of Acids • HCl(g) H2O H+(aq) + Cl-(aq) hydrogen ion • This is the same as: • HCl(g) H2O H3O+(aq) + Cl-(aq) hydronium ion The hydrogen ion (H+) is the same thing as the hydronium (H3O+) ion. • The hydrochloric acid, HCl is ionized or dissociated in water, so hydrochloric acid is an electrolyte. The name hydrochloric acid already implies that the acid, HCl or hydrogen chloride, is dissolved in water. Boiling Point • Occurs when the pressure of the vapor escaping from a liquid is equal to the atmospheric pressure. • The pressure exerted by the vapor is called the vapor pressure. • The normal boiling point of a liquid is the boiling point at 1 atm of pressure, which is the pressure at sea level. • The normal boiling point of water is 100oC, or 212oF. Pure Solvent Solution The rate of evaporation, and thus the vapor pressure, is less for a solution than for a solvent in the pure state. The greater the solute concentration, the less the vapor pressure. Therefore, the higher the boiling point. Boiling Point of Solutions • It doesn’t matter what the substance is, the more particles of solute are present, the more the boiling point will be increased, because boiling occurs from the surface and the presence of solute particles decreases the rate of particles escaping to the gas state. They hinder the boiling process. • This is called boiling point elevation The more solute particles there are, the higher the boiling point. For ionic compounds each ion counts as a particle, so the effect is greater. Freezing Point • Freezing occurs when the kinetic energy of molecules has been reduced sufficiently so the molecules can come together, forming the crystal structure of the solid. • The freezing point of water at 1 atm is 0oC or 32oF. • In aqueous solutions, the presence of solute particles interferes with the water molecules as they attempt to form the six-sided hexagonal crystalline structure. • The temperature has to be below the freezing point of the solvent for the solution to freeze. Freezing Point of Seawater • The freezing point of seawater is lower than that of water. • The ice which forms does not contain any solute particles, it is pure water. • As some ice forms the remaining water contains a higher solute concentration, so the freezing point is further decreased. • This is called freezing point depression. Practical Uses of Freezing Point Depression and Boiling Point Elevation • Salt is spread on snow to lower the freezing point of water, which is what snow is made up of. CaCl2, calcium chloride, is often used for this purpose. This avoids the roads to become slippery after the snow melts. • Ethylene glycol is antifreeze and is added to radiator water to lower the freezing point and raise the boiling point of water. This is protection against temperatures that are too high or too low. Acids, Bases, and Salts • Salts are ionic compounds. The hardness of water is related to ionic compounds or salts dissolved in water. • The acidity of soils determines how well plants grow. • Acid rain is a by product of industry and automobiles. This is harmful for living organisms. Acids, bases and salts • Acids have sour tastes • Changes litmus paper from blue to red • Acids react with metals releasing hydrogen gas • Acids neutralize bases forming water and salt Acids, bases and salts • Bases have bitter taste (caffeine) • Bases turn litmus paper blue • Basic solutions feel slippery on skin. • Bases neutralize acids forming water and salts Acids, bases and salts • Are there any similarities in the lists shown before? Acid - any substance that is a proton donor when dissolved in water. A proton is a hydrogen ion, H+ Hydronium ion This is the same as: H2O HCl H+(aq) + Cl-(aq) Bases • Many bases are ionic compounds which contain hydroxide ion (OH-) as the negative ion. • They produce OH- when dissolved in water: NaOH Na+(aq) + OH-(aq) Proton acceptor A proton is a hydrogen ion (H+) Acids, bases and salts • Other bases, like ammonia, NH3, will produce the OHonce it is dissolved in water, since a hydrogen ion gets transferred from a water molecule to the ammonia molecule. H+OHHydroxide ion Base - Any substance that is a proton acceptor when dissolved in water. Proton acceptor Acids, bases and salts • What happens when acids and bases mix? The H+ from the acid and the OH- from the base react to form water: H+ (aq) + OH-(aq) H2O(l) This is called neutralization Strong Acids • A strong acid is one that ionizes (dissociates) 100% in aqueous solution. • HNO3 H2O H+(aq) + NO3-(aq) 100% • Acids can be recognized because the formula begins with “H”. • Common strong acids include: HCl hydrochloric acid HBr hydrobromic acid HI hydroiodic acid H2SO4 sulfuric acid HNO3 nitric acid Weak Acids • Weak acids only partially ionize (dissociate) in aqueous solution. They only produce a small percentage of hydrogen ions (H+). H2O • HF not 100% H+(aq) + F-(aq) • A double arrow indicates an equilibrium, so the reaction occurs both ways, to the right and to the left. • The arrow to the left is longer because there is more of the non ionized acid present than of the ions at any given time. Weak Acids • Common weak acids include: HC2H3O2 acetic acid H2CO3 carbonic acid • HC2H3O2 H 2O H+(aq) + C2H3O2-(aq) In the case of acetic acid, only about 1% of the acid molecules ionize in aqueous solution. Strong Bases • Strong bases are those that completely (100%) ionize (dissociate) in aqueous solution. NaOH H2O Na+(aq) + OH-(aq) 100% • Common strong bases include: NaOH sodium hydroxide, common name lye, which is used to make soap. KOH potassium hydroxide Also the other Group IA and Group IIA hydroxides except for Be(OH)2 and Mg(OH)2. Weak Bases • Weak bases only partially ionize (dissociate) in aqueous solution. Mg(OH)2 Mg2+(aq) + 2OH-(aq) not 100% NH4+(aq) + OH-(aq) NH3(aq) + H2O not 100% The pH Scale • The strength of acids and bases is measured using the pH scale. • A neutral substance, like water, which is neither an acid nor a base, has a pH of 7. • Acidic solutions (acids dissolved in water) have a pH lower than 7. • Basic solutions (bases dissolved in water) have a pH higher than 7. Salts • When an acid reacts with a base a salt (ionic compound, except bases) is formed. Water is also formed: HCl + NaOH acid base 2HNO3 + Ca(OH)2 acid base NaCl + H2O salt water Ca(NO3)2 + 2H2O salt water • Neutralization always occurs 100% between an acid and a base even if they are weak. Importance of Salts • Salts are important in the diet, since we need them as electrolytes and as a source of certain elements. These are minerals. • Plants also require the elements that can be provided by certain salts and are added to plants as fertilizers. For example, K, N (supplied as nitrates, NO3-), and P (supplied as phosphates, PO43-). • Not all salts are soluble in water. Insoluble salts is what gives rise to hard water that ends up forming rings in your bathtub because when mixed with soap insoluble salts are formed. Practice Exercises • p. 294-296 Applying the Concepts: # 1, 2, 3, 4, 6, 7, 9, 10, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 Review Chapter 11 • • • • • • • • Water-polar molecule, undergoes H-bonding. Unusual characteristics of water molecule. Heterogeneous and Homogeneous (Solutions) mixtures. Saturated, unsaturated, and miscible solutions. Like dissolves like: Polar solvents like water dissolve polar molecules and ionic compounds. Non polar solvents, like Carbon Tetrachloride dissolve nonpolar solutes, like grease. Soap-Polar and non polar ends, dissolves in water and in oils and greases. How ionic compounds dissolve in water. Concentrated vs. dilute. % by mass and % by volume. • • • Solubility-maximum amt. of solute that can dissolve in a solvent at a given temperature. Solubility of ionic compounds usually increases with increasing T, solubility of gases decreases with increasing T. Electrolytes: contain ions in solution. Includes ionic compounds dissolved in water and also acids dissolved in water. Covalent compounds, including water, are non electrolytes. Review for Chapter 11 (cont.) • • • • • Ionization of strong and weak acids in aqueous solution (100% for strong acids, equilibrium for weak acids). (Strong acids are sulfuric, nitric, hydrochloric, hydrobromic, and hydroiodic acids. The rest are weak acids.) Ionization of strong and weak bases in aqueous solution.(100% for strong bases, equilibrium for weak bases). (Strong bases are the group IA (alkali metals) combined with hydroxide ion. What is vapor pressure-The pressure exerted by the vapor that evaporates from a liquid on the surface of the liquid. Vapor Pressure increases with temperature. What is boiling point. The temperature at which the vapor pressure of a liquid is equal to the atmospheric pressure. Boiling point elevation and freezing point depression in solutions. (The boiling point increases, the freezing point decreases). • Properties of acids and bases. • How ammonia, NH3 is a base because it reacts with water to form NH4+ and OH- in aqueous solution. • Neutralization reactions: Acid + Base react to produce salt (ionic compound) plus water. • The pH scale: 0-14. 7 is neutral, like water. Less than7 is acidic, higher than 7 is basic. • The importance of salts: They provide electrolytes to living organisms, including plants, animals and humans and they are a source of some essential elements.