2.4 Enzymes
advertisement

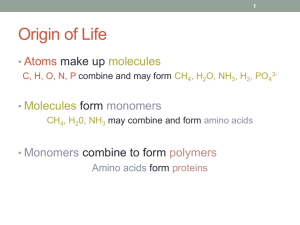
2.4 Enzymes Chemical Reactions Changes or transforms one set of substances into another • Breaking and Reforming Bonds • Example: O2 + 2 H2+ energy 2 H2O Reactants Products Some release energy (exothermic) Some absorb energy (endothermic) All Chemical Reactions…. Require some energy to get things going (to start breaking bonds) Activation Energy The energy needed to get a reaction started. Some chemical reactions often occur spontaneously. Others that absorb energy cannot occur without a source of energy. Role of Catalysts • Allow reaction to happen more readily • Speeds up the rate of the reaction • NOT used up in the reaction Enzymes Proteins that speed up chemical reactions within cells. Example: carbonic anhydrase CO2 + H2O H2CO3 Converts Carbon Dioxide to Carbonic Acid 10 million times faster than on its own by LOWERING THE ACTIVATION ENERGY NEEDED to complete the reaction How Enzymes Work? Brings reactants close together (in correct orientation) so reaction occurs much easier. Active Site is where the substrate bonds to the enzyme Regulation of Enzyme Activity The following affect the activity of enzymes: 1. Temperature • What do you expect the ideal temperature of activity to be? (37 degrees Celsius – normal body temperature) 2. pH • What effect would pH have on activity? Denature • What pH would you expect stomach enzymes (peptidase, lipase) to work most effectively at? – Acidic 3. Regulatory Molecules • Bind to enzymes to speed up or slow down their activity by turning them on or off. Minute Paper On a separate piece of paper do your best to answer the question (drawings work well!): What role do enzymes play in living things?