Origin of Life Atoms make up form
advertisement
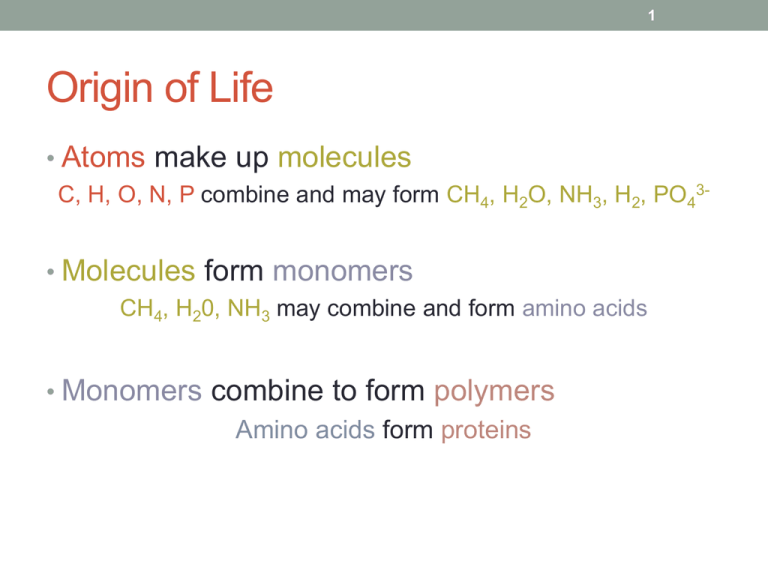
1 Origin of Life • Atoms make up molecules C, H, O, N, P combine and may form CH4, H2O, NH3, H2, PO43- • Molecules form monomers CH4, H20, NH3 may combine and form amino acids • Monomers combine to form polymers Amino acids form proteins 2 Definitions • ___________: a single unit molecule that may chemically bind to other molecules Ex. ___________________: building blocks of protein • ___________: a chain of repeated monomers Ex. _____________: a large molecule formed by linked monomers called amino acids 3 Enzymes: special type of protein • __________: a type of ________ that speeds up chemical _________ in living organisms without being _________ or destroyed. • Chemical reactions are synonymous with metabolic reactions in biology. • Are picky and only work on one molecule Example: catalase only works with H2O2 (hydrogen peroxide) 4 CHEMICAL REACTIONS Transfer of Energy Rearrangement of Atoms 5 Energy Definitions: • Ability to do work • Ability to move or change matter Energy comes in ______ forms. 6 Forms of energy • Light - sun • Heat - shivering • Mechanical - rub hands together • Electrical - shock metal door knob • Chemical - combustion 7 Chemical Reactions • Energy is transferred • Atoms are rearranged 8 Energy is Transferred First Law of Thermodynamics: The total amount of energy in an isolated system is constant (not increasing or decreasing). Energy cannot be created nor destroyed. 9 Rearrangement of Atoms __________: starting materials Product: newly formed substances : changes into A+BC+D Ex. HCl + KOH KCl + H2O 10 Energy in Chemical Reactions • Chemical bonds are formed and broken • Hydrogen Bonds, Ionic Bonds, Covalent Bonds • Products can either _______ or _______ energy • Endothermic Reaction • Exothermic Reaction • -gonic = suffix meaning energy 11 Chemical Bonds and Reactions • Are strong bonds easier or harder to break compared to weak bonds? And do they require more or less energy? • Therefore, weak bonds are _________ to break and require ________ energy to do so. • Do spontaneous reactions break ‘more stable’ or ‘less stable’ bonds? Why? 12 RULE OF THUMB The more strong bonds a molecule has, the more stable it is; consequently, more energy is needed to break down the molecule. 13 What is happening in this reaction? 14 How would you graph the diagram to the right? High Energy (heat) Low Time 15 Endergonic Reaction • Energy is absorbed • Unfavorable or _________________ • Products are less stable than reactants Ex 1. Photosynthesis 6CO2 + 6H2O C6H12O6 + 6O2 Ex 2. burning paper 16 What is happening in this reaction? 17 How would you graph the diagram to the right? High Energy (heat) Low Time 18 Exergonic Reaction • Energy is released • Favorable or spontaneous • Products more stable Ex. Respiration C6H12O6 + 6O2 6CO2 + 6H2O 19 Enzymes and Metabolic Reactions Endergonic Reaction Exergonic Reaction • Energy is _________ • Energy is __________ • Products have ______ • Products have ______ energy than reactants energy than reactants 20 Starting Chemical Reactions • How do endergonic and exergonic reactions begin? 21 Activation Energy • Activation energy (EA): the energy needed to ________ a reaction • Do spontaneous reactions require EA? • Do non-spontaneous reactions require EA? 22 Enzyme and EA (Activation Energy) 23 Role of Enzyme 24 Enzymes • protein that speeds up metabolic reactions • Catalysts: _______ the activation energy needed to start a chemical reaction How do enzymes speed up chemical reactions? 25 Importance of Enzymes • Allow organisms to maintain homeostasis • Without enzymes, reactions would occur too slowly for life to exist • Enzymes lower the activation energy needed for chemical reactions to occur = quicker reactions 26 Enzyme Specificity • Specificity- specific, particular, precise • Enzymes only work with certain _____________ or reactants. • ____________: substance or molecule which an enzyme acts on Amylase Starch H2O2 Catalase glucose 2H2O + O2 27 How are enzymes specific? 28 Enzyme Lock and Key Model 29 What effects the performance of enzymes? • Two things. Discuss with your table partner for a few moments. 30 31 32 THE END






