YouTube video 1:21
advertisement

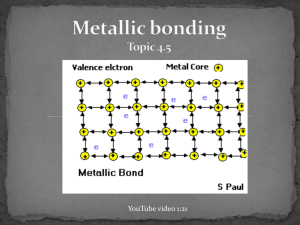
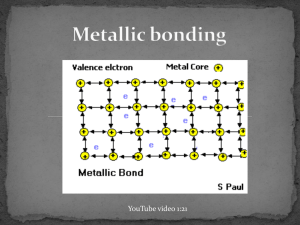
YouTube video 1:21 valence electrons detach from individual atoms since metals contain only 1-3 valence electrons and a low ionization energy bonding is non-directional no particular electron is confined to a particular metal cation leaves a close packed lattice of cations in a sea of delocalized negative electrons the electrostatic attraction between the cations and sea of electrons holds the metal together electrons act as a "glue" giving the substance a definite structure Characteristics strength of a metallic bond depends on… 1. number of delocalized valence electrons more delocalized electrons = greater electrostatic attraction (higher MP) 2. size of cation’s charge stronger the charge = greater electrostatic attraction (higher MP) 3. ionic radius when the metallic atom radius decreases, the sea of negative electrons becomes closer to the nucleus and there is a stronger attraction to them therefore, melting point decreases as you go down the periodic table the delocalized nature of the bonds, make it possible: conduct electricity for the atoms to slide past each other this means metals are malleable and ductile hammered into thin sheets or shapes without breaking drawn into wires ionic compounds would crack contain more than one metal and have enhanced properties such as greater strength resistance to corrosion enhanced magnetic properties greater ductility examples steel Fe and C -- high tensile strength but can rust brass Cu and Zn – looks cool! gold alloy, dental amalgam, and so on… metals are essential part of the world economy reinforcement of concrete, wires, cars, pipes… traded as commodities most common is iron which is in steel