Metallic Bonding Worksheet
advertisement

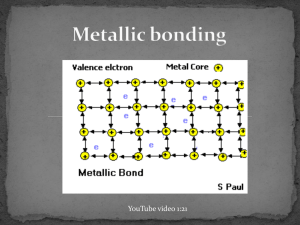
UNIT 6 - ELECTRICITY Section 6.1 - Conductivity METALLIC BONDING 6.1.a Metallic Bonding 6 https://youtu.be/9lDVDf9AKhQ or search: Metallic Bonding & Properties Tutorial [Now with Animations!] Metallic bonding is the ELECTROSTATIC ATTRACTION between METAL CATIONS (+) and MOBILE VALENCE ELECTRONS (–). Metals have Giant Structures “Giant Structures” refer to large, non-molecular arrangements of atoms. Examples of a “giant structures” are an ionic crystal lattices and covalent network solids. “Giant” implies that the structure is large, but in truth, variable numbers of atoms are involved. In a metal, atoms are packed closely together in an orderly manner. Ex. Elemental Sodium = sodium metal on its own A sodium atom on its own has a net charge of zero since the # of protons =the # of electrons. When a bunch of sodium atoms get together they form an orderly 3-D CRYSTAL LATTICE. But sodium’s valence electron DOESN’T stay attached to it’s “home atom”… Metallic Bonding Each metal atom gives up its VALENCE electrons to form cations (positive ions). These electrons do NOT belong to any particular particular metal atom therefore we say they are delocalized. They don’t have a specific “home”. These delocalized valence electrons are mobile which means they move freely in the space between the metal ions. UNIT 6 - ELECTRICITY Section 6.1 - Conductivity 6.1.a Metallic Bonding Q. Why do the metal ions give up their valence electrons to this “sea” of mobile, delocalized electrons? A. ① Metallic atoms have low electronegativities, which means they tend to lose their outer shell electrons easily. ② Once a metallic atom has lost its outer shell electron, it forms a positively charged ion called a cation. This produces electron configurations similar to the noble gases and hence a more stable state. stable noble gas electron configuration Ex. = a sodium cation NOTE: = a valence electron Only a few of the electrostatic interactions are shown in the figure above. Metallic bonding is the ELECTROSTATIC ATTRACTION ( ) between METAL CATIONS (+) and MOBILE, DELOCALIZED VALENCE ELECTRONS (–). UNIT 6 - ELECTRICITY Section 6.1 - Conductivity 6.1.a Metallic Bonding Another image showing the “sea” or “swarm” like nature of the valence electrons: The outer electrons are so weakly bound to the metal atoms that they are free to roam across the entire metal. Having “lost” their outer electrons, individual metal atoms are more like positive ions in a swarm of communal electrons. Physical Properties - Explanation ① Solid, high density Metals are packed tightly in layers. ② High melting and boiling points Metal ions are held together by their strong electrostatic attraction to the delocalized electrons between the ions. i.e. Metals are held together by strong metallic bonds. ③ Malleable and Ductile When a force is applied to a metal, the layers of atoms can slide over each other easily. UNIT 6 - ELECTRICITY Section 6.1 - Conductivity 6.1.a Metallic Bonding ③ Malleable and Ductile (continued) The metal does not break because the “sea” of mobile electrons is free to move within the structure, so they continue to attract the cations even when deformed. Malleable (can be hammered into sheets) Ductile (can be drawn into wires) UNIT 6 - ELECTRICITY ④ Metals are shiny: Section 6.1 - Conductivity Property - Lustre 6.1.a Metallic Bonding UNIT 6 - ELECTRICITY Section 6.1 - Conductivity 6.1.a Metallic Bonding ⑤ Good conductor of heat The movement of delocalized electrons allows heat to be conducted. When a metal is heated, the electrons at the heated end move faster, and they collide with neighbouring electrons with lots of energy. This makes the neighbouring electrons move more quickly as well, and soon all the electrons in the metal are moving quickly. ⑥ Good electrical conductor Metals conduct electricity when solid and when molten (melted). Current electricity is the movement of charged particles in a particular direction. This is possible in a metal because the delocalized, mobile electrons can move. As one electron moves away from its “home” atom, a second electron moves into the “hole” left behind by the first electron. If you put a positive charge at one end, and a negative charge at the other end of a copper wire, the electrons will flow in one direction… away from the negative (– charge repels e–‘s) and towards the positive (+ charge attracts e–‘s) Now watch the following video that shown an animation of how the delocalized electrons allow a current to flow in a wire. 6 What is a copper atom? Engineering Technology Simulation Learning Videos (1:44) https://youtu.be/O4lkxkbeo3s UNIT 6 - ELECTRICITY Section 6.1 - Conductivity 6.1.a Metallic Bonding Exercises: 1. Metals have delocalized valence electrons. a) What does it mean that the valence electrons are delocalized? b) In addition to being delocalized, the valence electrons of metals are also mobile. What does it mean that these electrons are mobile? 2. The valence electrons are said to form a “_________” (or “_______________”) of mobile delocalized electrons. fill in the blanks 3. a) Fill in the blanks stable ____________________ _________ electron configuration = a sodium _____________ = a _____________ electron b) Draw in between 5 and 10 electrostatic attractions using dashed lines. -------- c) These electrostatic attractions occur between stationary ________________________ and the mobile ______________________ ____________________________. d) What type of bond is formed by these electrostatic attractions? UNIT 6 - ELECTRICITY 4. Section 6.1 - Conductivity 6.1.a Metallic Bonding Use metallic bonding and the “sea of mobile electrons” model to explain why metals are a) good conductors of heat. b) good conductors of electricity. c) malleable and ductile d) lustrous 5. Compare and contrast the forces involved in ionic bonding, covalent bonding and metallic bonding. (When you are asked to “compare and contrast”, that means you should list the things that are similar or the same and the things that are different. UNIT 6 - ELECTRICITY Section 6.1 - Conductivity 6.1.a Metallic Bonding 6.1.a KEY ANSWERS: 1. Metals have delocalized valence electrons. a) What does it mean that the valence electrons are delocalized? The electrons are not bound to a “home atom” or bond. b) In addition to being delocalized, the valence electrons of metals are also mobile. What does it mean that these electrons are mobile? These delocalized electrons are “mobile”…free to move from one atom to another. 2. sea swarm The valence electrons are said to form a “_________” (or “_______________”) of mobile delocalized electrons. fill in the blanks 3. a) Fill in the blanks stable ____________________ _________ noble gas electron configuration ion = a sodium _____________ valence = a _____________ electron FOR EXAMPLE: b) Draw in between 5 or more electrostatic attractions using dashed lines. -------- c) cations These electrostatic attractions occur between stationary ________________________ and valence electrons the mobile ______________________ ____________________________. d) What type of bond is formed by these electrostatic attractions? a metallic bond UNIT 6 - ELECTRICITY Section 6.1 - Conductivity 6.1.a Metallic Bonding 6.1.a KEY 4. Use metallic bonding and the “sea of mobile electrons” model to explain why metals are a) When a metal is heated, the electrons at the heated end move faster, and they collide with neighbouring electrons with lots of energy. This makes the neighbouring electrons move more quickly as well, and soon all the electrons in the metal are moving quickly. b) The “sea” delocalized mobile electrons can move. As one electron moves away from its “home” atom, a second electron moves into the “hole” left behind by the first electron. If you put a positive charge at one end, and a negative charge at the other end of a copper wire, the electrons will flow in one direction… away from the negative (– charge repels e–‘s) and towards the positive (+ charge attracts e–‘s) good conductors of heat. good conductors of electricity. c) malleable and ductile The metal does not break because the “sea” of mobile electrons is free to move within the structure, so the mobile electrons continue to attract the cations even when the overall structure is deformed. d) lustrous Light rays bounce off the delocalized electrons. 5. Compare and contrast the forces involved in ionic bonding, covalent bonding and metallic bonding. (When you are asked to “compare and contrast”, that means you should list the things that are similar or the same and the things that are different. • All 3 types of bonding involve ELECTROSTATIC ATTRACTIONS between positive and negative charges. • In a covalent bond - the “positive things” are the nuclei of the bonded atoms - the “negative things” are the shared electrons in the bond • In an ionic bond - the “positive things” are cations - the “negative things” are anions • In a metallic bond - the “positive things” are the metal cations - the “negative things” are the delocalized valence electrons