between
advertisement

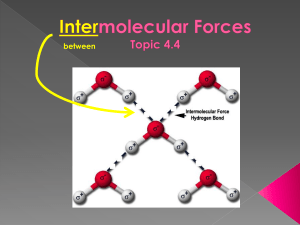
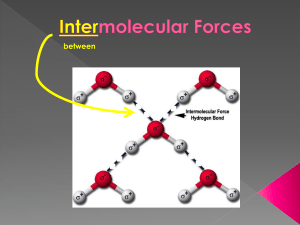
between Intra- › strong forces that hold the atoms in a molecule together e.g. – it takes 464 kJ/mol to break the H-O bonds within a water molecule › responsible for chemical properties burning, toxic, reactivity… Inter- › weak forces that holds molecules to one another e.g. – it takes only 19 kJ/mol to break the bonds between water molecules › the strength of the intermolecular forces determines the physical properties of the substance melting, boiling, solubility, conductivity, volatility 3 main types of intermolecular forces 1. London forces (also called dispersion forces, instantaneous induced dipole forces, or Van strength der Waals forces) increases 2. dipole-dipole forces (polar molecules) 3. a stronger type of dipole-dipole bonding called hydrogen bonding London Forces YouTube (:20) https://www.youtube.com/watch?v=S8QsL UO_tgQ occurs in all molecules, even diatomic molecules (HOFBrINCl) the distribution of electrons around an individual atom, at a given instant in time, may not be perfectly symmetrical › this can produce temporary/instantaneous dipole (polar molecule) › this can then induce a nearby molecule to be polar and therefore a very weak attraction between the two molecules and so on… › magnitude of the force depends on… 1. number of electrons and size of the electron cloud increases with more electrons, valence electrons are farther away from the nucleus and can be polarized more easily 2. shape of molecules molecules with shapes that have more contact area have greater forces between them than those that don’t these round shapes do NOT allow them to stick to one another this flat shape allows it to stick to one another better boiling point increases attractive forces between the positive end of one polar molecule and the negative end of another polar molecule the more polar the molecule, the greater the dipole-dipole force stronger than London forces YouTube Hydrogen Bonding (1:40) YouTube Hydrogen Bonding Video (:58) a specific type of dipole-dipole type interactions stronger than other dipole-dipole and London forces the hydrogen (H) in a molecule is intermolecularly bonded to a small, highly electronegative element (usually an N, O or F atom) on another molecule H-NOF the greater polarity of a molecule, the higher the boiling point › In HF, H is 2.1 and F is 4.0, difference of 1.9 › In HCl, H is 2.1 and Cl is 3.0, difference of 0.9 BP is 20°C BP is -85°C H-NOF ? › H2O vs. H2S? In H2O, H is 2.1 and O is 3.5, difference of 1.4 water molecules can hydrogen bond (and London forces) to each other BP is 100°C In H2S, H is 2.1 and S is 2.5, only a difference of 0.4 H2S can dipole-dipole bond (and London forces) to each other H-NOF ? BP is -60°C no H-NOF ? yes CH3OCH3 vs. CH3CH2OH ? -24°C dipole – dipole 78°C has hydrogen bonding NH3 vs. PH3 ? -33°C -87°C has hydrogen bonding dipole – dipole H-NOF ? CH3CH2 CH3 vs. CH3CHO vs. CH3CH2OH ? only London forces (VDW) low BP VDW & dipole-dipole medium BP VDW, D-D, & hydrogen bonding highest BP