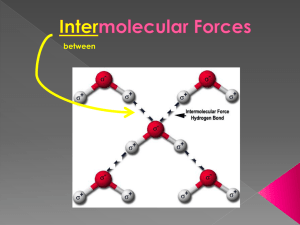
between

between
Intra-
›
› strong forces that hold the atoms in a molecule together
takes 464 kJ/mol to break the H-O bonds within a water molecule responsible for chemical properties
Inter-
›
› weak forces that holds molecules to one another
takes only 19 kJ/mol to break the bonds between water molecules the strength of the intermolecular forces determines the physical properties of the substance
melting, boiling, solubility, conductivity, volatility
3 main “types” of intermolecular forces
1.
London forces (also called dispersion forces or instantaneous induced dipole forces
2.
3.
dipole-dipole forces (polar molecules) a stronger type of dipole-dipole bonding called hydrogen bonding strength increases
* Van der Waals and London forces are often used interchangeably– more later on this.
van der Waals’ YouTube (:20) occurs in non-polar molecules the distribution of electrons around an individual atom, at a given instant in
time, may not be perfectly symmetrical
› this can produce temporary/instantaneous dipole (polar molecule)
› this can then induce a nearby molecule to be polar and therefore a very weak attraction between the two molecules
›
magnitude of the force depends on…
1.
number of electrons and size of the electron cloud
with more electrons, valence electrons are farther away from the nucleus and can be polarized more easily
2.
shape of molecules
molecules with shapes that have more contact area have greater forces between them than those don’t
this flat shape allows it to stick to one another better boiling point increases these round shapes do
NOT allow them to stick to one another
attractive forces between the positive end of one polar molecule and the negative end of another polar molecule must be in close proximity for the dipoledipole forces to be significant the more polar the molecule, the greater the dipole-dipole force stronger than London forces
YouTube Hydrogen Bonding (1:40)
YouTube Hydrogen Bonding Video (:58) a specific type of dipole-dipole type interactions
stronger than other dipole-dipole and
London forces the hydrogen ( H ) in a molecule is
intermolecularly bonded to a small, highly electronegative element (usually an N , O or F atom) on another molecule
H-NOF
the term London forces is used for instantaneous induced dipole – induced dipole force in non-polar molecules
Van der Waals is a more inclusive term, for all intermolecular attractions
Melting point (mp) - solid to liquid
Boiling point (bp) - liquid to gas
Volatility - how easily it is converted to gas
Conductivity (conducts electricity)
› depends on whether the substance contains freely moving charged particles
Solubility - solute’s ability to dissolve in solvent
the greater polarity of a molecule, the higher the boiling point
›
›
In HF, H is 2.1 and F is 4.0, difference of 1.9
In HCl, H is 2.1 and Cl is 3.0, difference of 0.9
BP is 20°C BP is -85°C
› H
2
O vs. H
2
S?
H-NOF ?
In H
2
O , H is 2.1 and O is 3.5, difference of 1.4
water molecules can hydrogen bond (and
London forces) to each other
BP is 100°C
In H
2
S, H is 2.1 and S is 2.5, only a difference of 0.4
H
2
S can dipole-dipole bond (and London forces) to each other
BP is -60°C H-NOF ?
no
H-NOF ?
yes
CH
3
OCH
3 vs. CH
3
CH
2
OH ?
H-NOF ?
-24°C 78°C dipole – dipole has hydrogen bonding
NH
3 vs. PH
3
?
-33°C -87°C has hydrogen bonding dipole – dipole
CH
3
CH
2
CH
3 vs. CH
3
CHO vs. CH
3
CH
2
OH ?
only London forces
(VDW) low BP
VDW & dipole-dipole medium BP
VDW, D-D, & hydrogen bonding highest BP