Review Chapter 6: Oxidation & Reduction Reactions Chemistry: The Molecular Nature of Matter,
advertisement
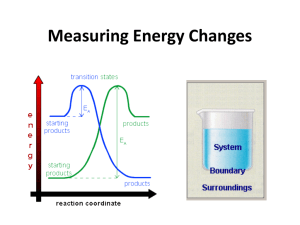
Review Chapter 6: Oxidation & Reduction Reactions Chemistry: The Molecular Nature of Matter, 6th edition By Jesperson, Brady, & Hyslop Chapter 7 Concepts Be familiar with Potential and Kinetic Energy Potential Energy changes as bonds break and form Average KE is proportional to Temperature Conservation of Energy Define system vs surroundings Track direction of heat transfer between system and surroundings Heat capacity calculations Specific heat calculations Exothermic vs Endothermic Heats of reactions: Calorimetry problems, qv vs qp First Law of Thermodynamics Define Enthalpy Enthalpy calculations: Hess’s law ΔHf ΔHrxn 2 Memorize Total = Potential + Energy Energy Kinetic Energy E = Efinal – Einitial T (K) Avg KE = ½ mvavg2 A calorie (cal) Energy needed to raise the temperature of 1 g H2O by 1 °C 1 cal = 4.184 J (exactly) 1 kcal = 1000 cal 1 kg m2 1 kcal = 4.184 kJ 1J 1 Cal = 1000 cal = 1 kcal s2 1 kcal = 4.184 kJ H°reaction = Sum of all H°f of all of the products q = C × t q = s m t Work = –P × V E = q + w H = E + PV – Sum of all H°f of all of the reactants Endothermic & Exothermic Surroundings PE heat System PE increases as bonds break ENDOTHERMIC Heat + Reactants Products Products have stronger bonds and therefore a higher potential energy then Reactants. Heat is absorbed by the system. ΔE + ΔH + PE decreases as bonds form ENDOTHERMIC Heat absorbed Reactants Heat released Products EXOTHERMIC EXOTHERMIC System heat Surroundings Reactants Products + heat Products have weaker bonds and therefore a lower potential energy then Reactants. Heat is released by the system. ΔE - ΔH - System & Surroundings heat qsystem = –qsurroundings System Surroundings Constant Volume qv ΔE Constant Pressure qp ΔH Open system Closed system Hess’s Law Hess’s Law of Heat Summation • Going from reactants to products • Enthalpy change is same whether reaction takes place in one step or many Reactants Path 2 step b Path 1 Products Path 2 step b Sum of Enthalpies from steps in path 2 = Path 1 Enthalpy Manipulating Thermochemical Rxns aA (state) + bB (state) cC (state) + dD (state) 1. 2. 3. ΔH When equation is reversed, sign of H°rxn must also be reversed. If all coefficients of equation are multiplied or divided by same factor, value of H°rxn must likewise be multiplied or divided by that factor Formulas canceled from both sides of equation must be for substance in same physical states Strategy for Adding Thermochemical Rxns 1. 2. 3. 4. Choose most complex compound in equation for one-step path Choose equation in multi-step path that contains that compound Write equation down so that compound is on appropriate side of equation has appropriate coefficient for our reaction Repeat steps 1 – 3 for next most complex compound, etc. 5. Choose equation that allows you to cancel intermediates multiply by appropriate coefficient 6. Add reactions together and cancel like terms 7. Add energies together, modifying enthalpy values in same way equation modified If reversed equation, change sign on enthalpy If doubled equation, double energy