Name______________ Block_____Unblock____
advertisement
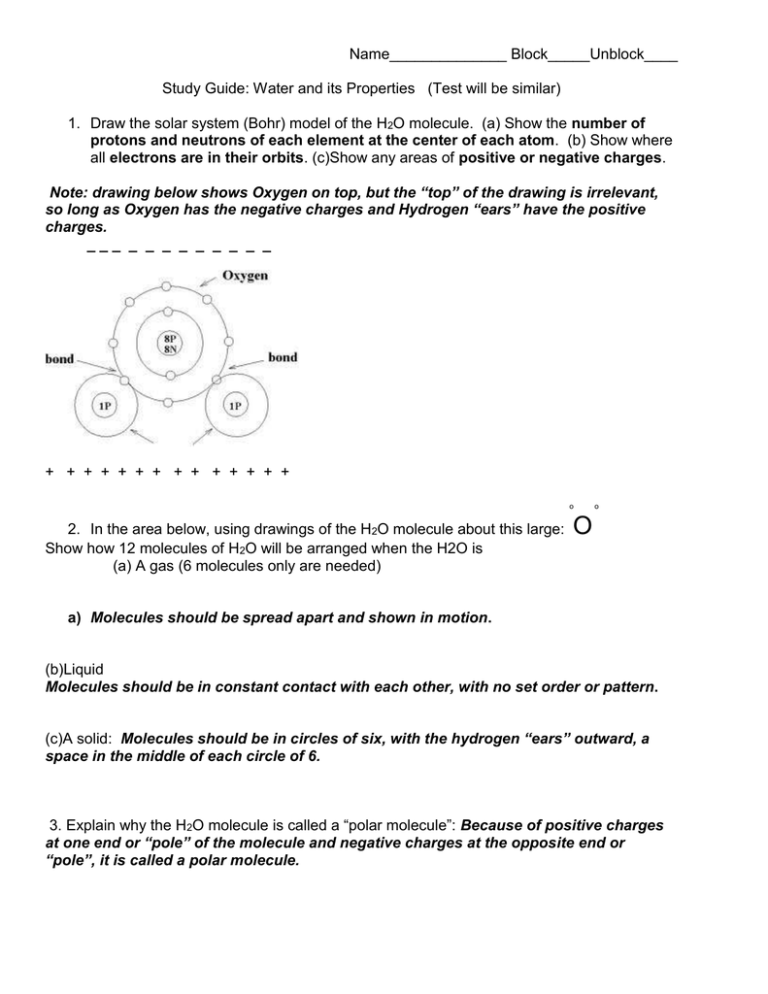
Name______________ Block_____Unblock____ Study Guide: Water and its Properties (Test will be similar) 1. Draw the solar system (Bohr) model of the H2O molecule. (a) Show the number of protons and neutrons of each element at the center of each atom. (b) Show where all electrons are in their orbits. (c)Show any areas of positive or negative charges. Note: drawing below shows Oxygen on top, but the “top” of the drawing is irrelevant, so long as Oxygen has the negative charges and Hydrogen “ears” have the positive charges. ___ _ _ _ _ _ _ _ _ _ + + + + + + + + + + + + + + o 2. In the area below, using drawings of the H2O molecule about this large: Show how 12 molecules of H2O will be arranged when the H2O is (a) A gas (6 molecules only are needed) o O a) Molecules should be spread apart and shown in motion. (b)Liquid Molecules should be in constant contact with each other, with no set order or pattern. (c)A solid: Molecules should be in circles of six, with the hydrogen “ears” outward, a space in the middle of each circle of 6. 3. Explain why the H2O molecule is called a “polar molecule”: Because of positive charges at one end or “pole” of the molecule and negative charges at the opposite end or “pole”, it is called a polar molecule. 4. Define the following, and provide an example Capillary action of water Water moving up or through small tubes or fibers. example A drop of water spreading on a paper towel Surface tension of water: Strong bonds at the surface of water, allows water striders to walk on them, hurts you if you do a belly flop. Immiscibility of water Water not mixing with nonpolar liquids example Water won’t mix with oil. Water as a solvent Water dissolving a solid or a gas. Example Water dissolving sugar. 5. Three factors that are special to water but not listed above affected the “drops on a penny” acitivity. What are the 3 factors, and how did they let students build up a large mound of water on the penny? Adhesion – where the water molecules stick to the copper penny molecules. Cohesion – where the water molecules stick to other water molecules on the copper penny Surface tension – where the water molecules at the top of the mound of water don’t allow an overflow to occur. 5. Fill in the blanks below for the states of matter of H2O and its transition verb. Starting state Transition Word Ending State A. ___solid(ice)_ __Melt_ ___liquid_ B. __liquid freeze solid ____________ C. __liquid evaporate gas ___________ __ D. __gas condense liquid ___________ __ E.solid sublimate gas ____________ F. gas depose solid 7. What is special about water’s specific heat, and how does it affect climate? Water has a very high specific heat, meaning it takes a lot of heat energy to change its temperature. This means that climate is moderated by large bodies of water, like oceans. So, places near the ocean tend to have warmer winters and cooler summers than places at similar latitudes but inland. 8. Imagine that a rogue H2O molecule was part of a gang in South Carolina that destroyed homes, farms, offices, and stores while also causing the deaths of wild animals, farm animals, pets, and people. Using a separate sheet of paper, prepare a poster warning people about the dangers of this molecule when in an unruly gang. Draw a wanted poster, after all, floods and tsunamis have killed lots of people.





