Study Guide for the Matter and Elements Quiz
advertisement
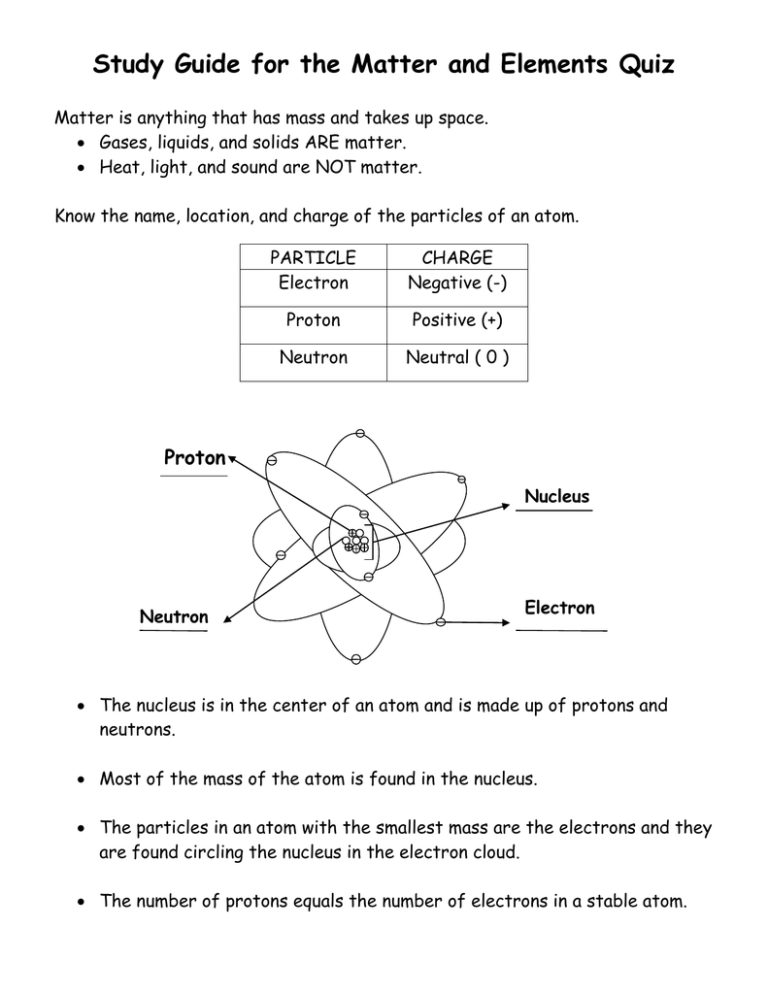
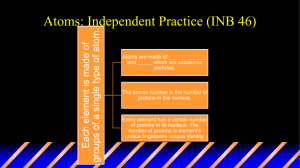
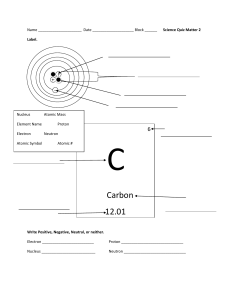
Study Guide for the Matter and Elements Quiz Matter is anything that has mass and takes up space. Gases, liquids, and solids ARE matter. Heat, light, and sound are NOT matter. Know the name, location, and charge of the particles of an atom. PARTICLE Electron CHARGE Negative (-) Proton Positive (+) Neutron Neutral ( 0 ) Proton Nucleus Neutron Electron The nucleus is in the center of an atom and is made up of protons and neutrons. Most of the mass of the atom is found in the nucleus. The particles in an atom with the smallest mass are the electrons and they are found circling the nucleus in the electron cloud. The number of protons equals the number of electrons in a stable atom. Elements An element is matter made up of one type of atom. Some of the most common elements that make up a large portion of our Earth, living matter, atmosphere and oceans are listed below. H C N O Hydrogen Carbon Nitrogen Oxygen Mg Al Si K Magnesium Aluminum Silicon Potassium Fe Ca Na Iron Calcium Sodium Two of the elements commonly found in our bodies and the oceans are hydrogen and oxygen. Using the Periodic Table Elements are represented by symbols in the periodic table. Know the parts of the element ‘square’ from the periodic table. Element Name Atomic Number OR Number of Protons Calcium 20 Ca 40.078 Element Symbol Atomic Mass Remember to always review all of the notes and handouts