Study Guide for Water and Its Properties Test
advertisement
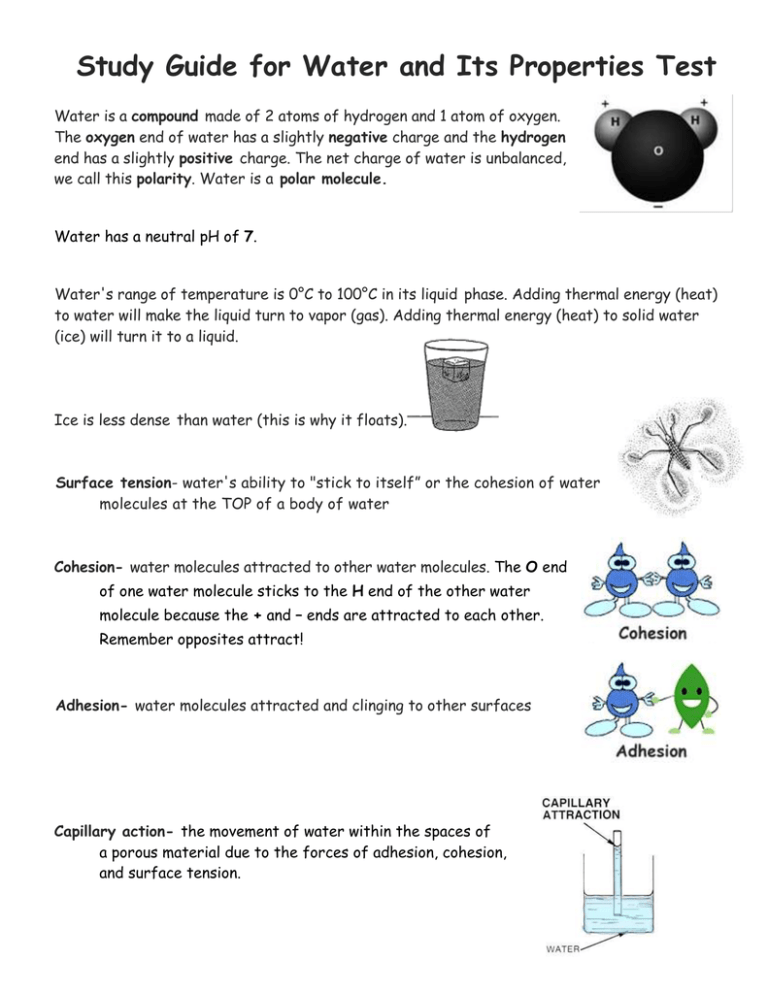
Study Guide for Water and Its Properties Test Water is a compound made of 2 atoms of hydrogen and 1 atom of oxygen. The oxygen end of water has a slightly negative charge and the hydrogen end has a slightly positive charge. The net charge of water is unbalanced, we call this polarity. Water is a polar molecule. Water has a neutral pH of 7. Water's range of temperature is 0°C to 100°C in its liquid phase. Adding thermal energy (heat) to water will make the liquid turn to vapor (gas). Adding thermal energy (heat) to solid water (ice) will turn it to a liquid. Ice is less dense than water (this is why it floats). Surface tension- water's ability to "stick to itself” or the cohesion of water molecules at the TOP of a body of water Cohesion- water molecules attracted to other water molecules. The O end of one water molecule sticks to the H end of the other water molecule because the + and – ends are attracted to each other. Remember opposites attract! Adhesion- water molecules attracted and clinging to other surfaces Capillary action- the movement of water within the spaces of a porous material due to the forces of adhesion, cohesion, and surface tension. Miscibility- the ability of a liquid to mix with another liquid Solubility- the ability of a solid to dissolve in a liquid Solute- substance that is being dissolved Solvent- substance into which the solute dissolves (Water is a universal solvent because it dissolves more substances than any other compound.) Surfactant- compounds that lower the surface tension of a liquid. Soap is an example of a surfactant. Water cycle- the constant movement of water from earth's surface into the atmosphere and back again Evaporation- the process where water changes from a liquid to a vapor; this is the opposite process from condensation. Precipitation- water droplets that fall from the clouds to the Earth Condensation- the process where water vapor cools and changes back into a liquid; this is the opposite process from evaporation. ** Know the 3 states of matter/stages of the water cycle and give an example of each: evaporation- liquid to gas- water leaving a cup left outside condensation- gas to liquid – clouds and dew precipitation- liquid or solid form- rain, snow, sleet, hail Remember to always review all of the notes and handouts!



