CHM 120 CHAPTER 16 KINETICS: Rates and Mechanisms of Chemical
advertisement

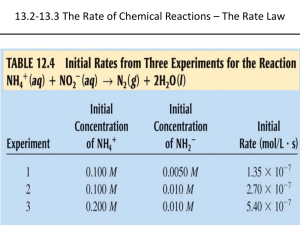
CHM 120 CHAPTER 16 KINETICS: Rates and Mechanisms of Chemical Reactions Dr. Floyd Beckford Lyon College CHEMICAL KINETICS • Two factors control the outcome of chemical reactions: 1. Chemical Thermodynamics 2. Chemical Kinetics • Chemical Kinetics: study of rates of chemical reactions and mechanisms by which they occur • A reaction may be spontaneous but does not occur at measurable rates REACTION RATES • Rate of reaction describes how fast reactants are used up and products are formed • There are 4 basic factors that affect reaction rates (i) Concentration (ii) Physical state (iii) Temperature (iv) Catalysts • For every reaction the particles must come into intimate contact with each other • High concentrations by definition implies that particles are closer together (than dilute solutions) • So rate increases with concentration • The degree of intimacy of particles obviously depends on the physical nature of the particles • Particles in the liquid state are closer than in the solid state • Likewise, particles in a finely divided solid will be closer than in a chunk of the solid • In both situations, there is a larger surface area available for the reaction to take place • This leads to an increase in rate • Temperature affects rate by affecting the number and energy of collisions • So an increase in temperature will have the effect of increasing reaction rate • Rate of reaction is typically measured as the change in concentration with time • This change may be a decrease or an increase • Likewise the concentration change may be of reactants or products concentration change Rate = time change in [products] in [reactants] Rate = ______________ = ______________ change in time change in time • Rate has units of moles per liter per unit time - M s-1, M h-1 • Consider the hypothetical reaction aA + bB cC + dD • We can write Rate of = - 1 [A] = - 1 [B] = a t reaction b t 1 [C] = 1 [D] c t d t • Note the use of the negative sign - rate is defined as a positive quantity - rate of disappearance of a reactant is negative 2N2O5(g) 4NO2(g) + O2(g) Rate of = - 1 [N2O5] = 1 [NO2] = reaction 2 4 t t [O2] t C2H4(g) + O3(g) C2H4O(g) + O2(g) • Rate may be expressed in three main ways: 1. Average reaction rate: a measure of the change in concentration with time 2. Instantaneous rate: rate of change of concentration at any particular instant during the reaction 3. Initial rate: instantaneous rate at t = 0 - that is, when the reactants are first mixed RATE LAW • Consider the following reaction aA + bB products • Rate of reaction changes as concentration of reactants change at constant temperature • RATE LAW: equation describing the relationship between concentration of a reactant and the rate Rate = k[A]m[B]n where k is called the rate constant • m, n are called reaction orders - they indicate the sensitivity of the rate to concentration changes of each reactant • NOTE: the orders have nothing to do with the stoichiometric coefficients in the balanced overall equation • An exponent of 0 means the reaction is zero order in that reactant - rate does not depend on the concentration of that reactant • An exponent of 1 rate is directly proportional to the concentration of that reactant - if concentration is doubled, rate doubles - reaction is first order in that reactant • An exponent of 2 rate is quadrupled if the concentration of that reactant is doubled - reaction is second order in that reactant • The overall reaction order is the sum of all the orders Rate = k[A][B]0 m = 1 and n = 0 - reaction is first order in A and zero order in B - overall order = 1 + 0 = 1 - usually written: Rate = k[A] • Remember: the values of the reaction orders must be determined from experiment; they cannot be found by looking at the equation DETERMINATION OF THE RATE LAW • The method of initial rates may be used - involves measuring the initial rates as a function of the initial concentrations - avoids problems of reversible reactions - initially there are no products so they cannot affect the measured rate • In this method the experiments are chosen so as to check the effect of a single reactant on the rate THE RATE CONSTANT 1. The units of k depends on the overall order of reaction 2. The value of k is independent of concentration and time 3. The value refers to a specific temperature and changes if we change temperature 4. Its value is for a specific reaction THE INTEGRATED RATE EQUATION • This is the equation that relates concentration and time • Consider a first-order reaction aA products Rate = k[A] conc. after time = t initial conc. ln [A]t [A]0 = -kt time [A]t = -kt log 2.303 [A]0 • The equation may be written in the form for a linear plot log [A]t -kt = + log [A]0 2.303 • A plot of log [A]t vs. t is linear plot with slope = -k/2.303 • Note that this plot gives a straight line ONLY if the reaction is first-order Half-life • The half-life, t1/2, is defined as the time it takes for the reactant concentration to drop to half its initial value t1/2 = ln 2 = 0.693 k k • Note: the half-life for a first order reaction does not depend on the initial concentration • The value of the half-life is constant Second order reactions • Consider a reaction that is 2nd order in reactant A and 2nd overall aA products and Rate = k[A]2 1 = kt + 1 [A]0 [A]t • A plot of 1/[A]t vs. t gives a straight line with slope = k t1/2 1 = k[A]0 RATES AND TEMPERATURE • Recall that temperature is the only factor that affects the rate constant • In general rates increase with temperature activation energy -(Ea/RT) k = Ae rate constant absolute temperature constant (related to collision frequemcy) • This is ARRHENIUS’ EQUATION • Can be arranged in the form of a straight line ln k = (-Ea/R)(1/T) + ln A • Plot ln k vs. 1/T slope = -Ea/R If T increases REACTION SPEEDS UP Ea/RT decreases k increases -Ea/RT increases e-Ea/RT increases • Another form of Arrhenius’ equation: ln k2 k1 =- Ea R 1 - 1 T2 T1 COLLISION THEORY: a reaction results when reactant molecules, which are properly oriented and have the appropriate energy, collide • The necessary energy is the activation energy, Ea • Not all collisions leads to a reaction • For effective collisions proper orientation of the molecules must be possible TRANSITION STATE THEORY • During a chemical reaction, reactants do not suddenly convert to products • The formation of products is a continuous process of bonding breaking and forming • At some point, a transitional species is formed containing “partial” bonds • This species is called the transition state or activated complex • The transition state is the configuration of atoms at the maximum of the reaction energy diagram • The activation energy is therefore the energy needed to reach the transition state • Note also that the transition state can go on to form products or break apart to reform the reactants REACTION MECHANISMS • MECHANISM: the step-by-step pathway by which a reaction occurs • Each step is called an elementary step NO2(g) + CO(g) NO(g) + CO2(g) Mechanism: NO2(g) + NO2(g) NO(g) + NO3(g) NO3(g) + CO(g) NO2(g) + CO2(g) • NO3 is a reaction intermediate • Elementary reactions are classified by the molecularity A B + C unimolecular A + B C + D bimolecular A + 2B E termolecular • Termolecular reactions are very unlikely • For ANY SINGLE ELEMENTARY REACTION – reaction orders are equal to the coefficients for that step A + B kelem C + D Rate = kelem[A][B] • The slow step is called the rate-determining step (RDS) • A reaction can never occur faster than its slowest step 1. Overall reaction = sum of all elementary steps 2. The mechanism proposed must be consistent with the rate law NO2(g) + NO2(g) NO3(g) + CO(g) NO2(g) + CO(g) k1 k2 NO(g) + NO3(g) NO2(g) + CO2(g) NO(g) + CO2(g) • There may be more than one plausible mechanism • The experimentally determined reaction orders indicate the number of molecules of the reactants - in the RDS (if it occurs first) - the RDS and any fast steps before it CATALYSIS • Reaction rates are also affected by catalysts • Catalyst: a substance that increases the rate of a reaction without being consumed in the reaction • Catalysts work by providing alternative pathways that have lower activation energies •A catalyst may be homogeneous or heterogeneous • Homogeneous: catalyst and reactants are in the same phase 2Ce4+(aq) + Tl+(aq) Mn2+ 2Ce3+(aq) + Tl3+(aq) • Heterogeneous: catalyst in a different phase • Typically: a solid in a liquid • An important example: catalytic converters in automobile - convert pollutants to CO2 H2O, O2, N2 - usually Pt, Pd, V2O5, Cr2O3, CuO • Cars must use unleaded fuels – lead poisons the catalytic bed