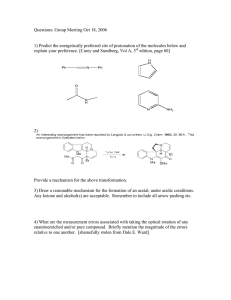
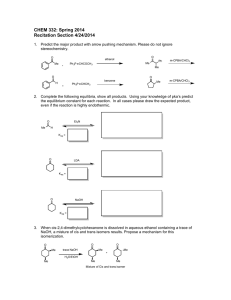
Developing Critical Thinking Problems for Organic Chemistry Ray A. Gross, Jr.

Developing Critical Thinking
Problems for Organic
Chemistry
Ray A. Gross, Jr.
Prince George’s Community
College
1
January 10, 2007
2
Thoughts About Students
• They do not spend enough time working problems.
• They need to be taught (learn) how to reason.
• One way to force them to reason is to give them problems they have not seen before.
3
Nature of the Problems
• Focused on developing reasoning skills
•
Solvable by applying course content
•
The amount of content is limited
4
Kinds of Problems Available
• Spectroscopy
• Synthesis
• Degradation*
5
Concept of Degradation
A compound of unknown structure is broken down into smaller pieces, which can be reassembled in a logical way to determine the structure.
6
Example of Ozonolysis
X unknown hydrocarbon
(1) O
3
(2) Zn/H
+
O
CH
3
CH
2
CCH
3
+
O
CH
3
CH
2
CH remove oxygen
HCCH
2
CH
3
CH
3
CH
2
CCH
3 analyte
CH
3
CH
2
CCH
3
+
CH
3
CH
2
CH anathons
7
C
7
H
14 analyte
O
3
(R)
C
4
H
8
O ketone
+
+
C
3
H
6
O aldehyde
C
4
H
8 anathons
C
3
H
6
Cleavage of a double bond yields two carbonyl compounds, aldehydes or ketones.
8
Some Aldehydes may be identified by their molecular formulas.
CH
2
O
= molecular formula
O
H
C
H structural formula
H
C
H anathon
9
Some Aldehydes may be identified by their molecular formulas.
C
2
H
4
O molecular formula
=
CH
3
O
C
H structural formula
CH
3
C
H anathon
10
Consider the following reaction.
C
3
H
6
O
3
(R)
CH
2
O + C
2
H
4
O
Given only molecular formulas.
11
Anathons are like building blocks.
O
3
C
3
H
6
CH
2
O + C
2
H
4
O
(R)
O
H
C
H
+
O
CH
3
CH
H
C
H
C
CH
3
H propene H
C
H
+
CH
3
CH
12
CH
3
CH
3
CH
2
C CHCH
2
CH
3
(1) O
3
(2) Ag
2
O
O
CH
3
CH
2
CCH
3 ketone
+
O
CH
3
CH
2
C O H acid
(1) O
3
(2) Zn/H
+
O
CH
3
CH
2
CCH
3
+ ketone
O
CH
3
CH
2
CH aldehyde
Ketones remain ketones
Aldehydes are oxidized to acids
13
C
7
H
14
Same reactions using molecular formulas.
(1) O
3
(2) Ag
2
O
(1) O
3
(2) Zn/H
+
C
4
H
8
O +
C
3
H
6
O
2
C
4
H
8
O + C
3
H
6
O
14
Identify aldehydes and ketones.
C
7
H
14
(1) O
3
(2) Ag
2
O
(1) O
3
(2) Zn/H
+
C
4
H
8
O +
C
3
H
6
O
2 no change
C
4
H
8
O ketone
+ plus 1 O
C
3
H
6
O aldehyde
Result: A four-carbon ketone and a three-carbon aldehyde.
15
Place C atoms in logical locations to find anathons.
C
7
H
14
C
4
H
8
O ketone
C
3
H
6
O aldehyde anathons
Concept
• Degradation of an unknown leads to products.
• The structure of each degradation product can be determined directly or indirectly from the two kinds of ozonolysis.
• The products can then be converted into anathons, which are connected in a logical way to produce the starting compound.
17
Producing Solvable Problems
• Find structures that are deducible from the two kinds of ozonolysis reactions.
• Convert them into anathons.
• Join anathons to make unknowns.
18
Structures from Molecular Formulas
(R)
CH
2
O
C
2
H
4
O
C
3
H
4
O
(O)
CO
2
C
2
H
4
O
2
R product
O
H C H
O
CH
3
CH
O
C
3
H
4
O
19
(R)
CH
2
O
C
2
H
4
O
C
3
H
4
O
Remove Oxygen to form Anathons
(O)
CO
2
C
2
H
4
O
2
R product
O
H C H
O
CH
3
CH
O
C
3
H
4
O
Anathon
H C H
CH
3
CH
20
Join Anathons to Create Unknowns
Anathon
H C H
CH
3
CH
Unknown
H
2
C CHCH
3
H
2
C
CH
3
CH
Internal Anathons
• When a molecule is cleaved in two places, the middle compound produces an internal anathon.
22
Example of an Internal Anathon
H
2
C CHCH
2
CH =CHCH
3
O
3
(O)
O
3
(R)
CO
2
+ C
3
H
4
O
4
+ C
2
H
4
O
2
CH
2
O + C 3
H
4
O
2
+ C
2
H
4
O
HCH terminal anathon
HCCH
2
CH CH
3
CH internal anathon terminal anathon
23
Carbon-Carbon Triple Bonds
• Ozonolysis by either workup gives two carboxylic acids.
24
Cleavage of a Triple Bond
O
3
(O)
CH
3
C CCH
2
CH
3
C
5
H
8
O
3
(R)
CH
3
CO
2
H + CH
3
CH
2
CO
2
H
CH
3
C O
2
H + CH
3
CH
2
C O
2
H
A triple bond in the analyte leads to two new hydrogen atoms and four new oxygen atoms.
25
2 new H atoms = triple bond two caboxylic acids
O
3
(R)
C
5
H
8
C
2
H
4
O
2 +
C
3
H
6
O
2
CH
3
C CCH
2
CH
3
Anathons from triple bonds have three bonding sites, and those from double bonds have two.
26
Anathons Readily Determined by their
Ozonolysis Products
• 11 Terminal Anathons
• 11 Interior Anathons
• 3 Either Terminal or Interior
306 unique unknown hydrocarbons can be produced from the 22 anathons. All of them are solvable by the techniques shown.
27
Example of Terminal/Interior Anathon terminal anathon
A second terminal anathon is bonded here.
Three terminal anathons are bonded as indicated.
interior anathon
28
Examples of the Unknowns
CH
3
CH
2
CH CHCH
2
CH C(CH
3
)
2
CH
3
CH
3
CH
2
CH C C
CH
2
CH
3
CHC CCH
2
CH
3
29
Problems Created that:
• Students have never seen before
• Require a minimum amount of course content
• Require students to apply logic and reasoning
(synthesis, analysis and evaluation)
• Increase in complexity over the semester
• Allow the instructor to give each student a unique problem each week
30
The approach described might be of use in other chemistry courses or disciplines.
31
Problems of this type are available in the following references.
Chemical Educator 2006, 11, 372-377.
Journal of Chemical Education 2006, 83, 604-609.
32