Chapter 1 I. Definitions A. Anatomy
advertisement

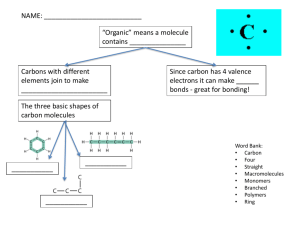
Chapter 1 I. Definitions A. Anatomy B. Physiology C. Microscopic vs. Gross Anatomy 1. Cytology 2. Histology D. Cell E. Tissue F. Organ G. Homeostasis- Every cell is made of, and manipulates, chemicals. Every cell, tissue, organ and various functional chemicals require a specific range of environmental conditions to function properly (ex., pH, temp, ion concentration). Those ranges often risk being disrupted, and cells constantly work to maintain and restore them. See example in book: response to rising heat during exercise. II. Levels of organization in the body Molecular/chemical- ex., proteins, DNA, calcium, phospholipids, etc. --> Cellular --> Tissue --> Organ --> Organ System (see pp. 9-10, fig 1-3 for organ systems of the body) --> Organism III. Homeostasis- General Overview A. General Control Mechanisms 1. Autoregulation- localized group of cells/tissue "fixes its own problem": examples: -cells need more O2, release vasodilators causing local blood vessels to expand and bring more blood with O2 -injured cells release chemicals to start the clotting process and attract immune cells 2. Extrinsic regulation- a broader, larger scale response to maintain homeostasis. Hormones &/or neurotransmitters are released in response to a change in homeostasis. They are released by an organ and sent throughout the body, not so localized. Often, hormones released by one organ affects the activities of distant organ/s, and may affect different organs in different ways. Examples: -blood sodium concentrations rise, a hormone released by a gland in the brain causes kidneys to retain water -blood glucose concentrations rise, the pancreas releases insulin. Insulin causes most body cells to take in glucose and use it for energy, causes fat cells to store fat (triglycerides), and causes liver & muscle cells to store glucose. B. Regulatory mechanisms employ 3 steps: 1. Sense the change via a receptor (ex: hypothalamus in the brain monitors temperature, senses change in temp) 2. A control center will process the information and respond (ex.: hypothalamus directs the release of some chemicals that will help restore temperature) 3. Effectors will respond to the chemical commands of the control center (ex: surface blood vessels relax [dilate], allowing more blood near the surface to release heat) C. Chemicals released to maintain/restore homeostasis can work in one of two ways: 1. Negative feedback (most common): ex., blood glucose rises, insulin causes changes that decrease blood glucose levels 2. Positive feedback: ex., blood clotting. When vessels are damaged, chemicals are released by local cells to start the clotting process. As the process starts, it induces cells to release more chemicals that will accelerate the process, and so on, until the vessel is patched. IV. Anatomical Landmarks, Body Cavities & Slices- use Lab Exercise 2 as a guideline. Chapter 2 I. Atoms & Molecules A. The number of electrons an element has determines how reactive it will be. Some elements tend to give up electrons, some tend to take them on. The one that lost the electron carries a positive charge, the one that took it carries a negative charge. Charged atoms are called ions. Positive ion = cation, negative ion = anion. Some elements give up or take on more than one electron. For example, calcium tends to lose 2 electrons and exists as a Ca 2+ cation. B. Molecules & Bonding 1. Ionic bonding: one atom gives up an electron to another atom. Both become more stable, but also become charged. Now they are attracted to each other because of their opposing charges. Table salt, or sodium chloride, is a classic example. Ionically bonded substances can easily dissociate in water to become their component ions. 2. Covalent bonding: atoms share electrons. The electrons they share zip around both of their nuclei. Covalent bonds are stronger than ionic bonds, and covalently bonded molecules do not tend to dissociate in water. Atoms that share 1 pair of electrons are single-bonded; 2 pairs, double-bonded; 3 pairs, triple-bonded. Sometimes, the atoms share the electrons equally. But sometimes, the atoms of a covalent bond have different electron affinities, and the electrons spend more time around one of the atoms than the other. If the electrons in a molecule tend to spend more time around certain atoms than others, then the atom/s with the extra electron time take on a slight negative charge, and the others a slight positive charge. We say that they are polar, or have polarity. 3. Hydrogen bonding: weak associations between polar molecules. The slightly negative end of one polar molecule is attracted to the slightly positive end of another. Important when many polar molecules interact through hydrogen bonding. The surface tension of water is a result of H-bonding: O hogs electrons and takes on a slight negative charge, while H takes on a slight positive charge. O and H of adjacent molecules are attracted to one another. H-bonding between base pairs in DNA holds the two strands together. Proteins are huge molecules that have polar sections. The unique folding of each protein is, in part, determined by H-bonding between sections of the protein strand. Ionically bonded ions tend to dissociate (separate) in water, because the slightly positive H's of water surround anions, and the slightly positive O's surround cations. See the explanation of pH below for an example of the dissociation of ionically bound substances in water. II. Reactions & Enzymes A. Background: energy 1. Kinetic energy: the energy of movement. Includes a ball flying through the air, or a molecule bouncing around in space. Heat is a form of kinetic energy: the random movement of molecules/atoms (the faster they go, the hotter it gets). Kinetic energy also includes a rubber band flying through the air toward the back of your little sister's head. 2. Potential energy: stored energy. When you stretch the rubber band back and aim it at your sister, you know that there is stored energy in that rubber band, because when you let go it will move, and will do work (startle your sister). Energy is stored in chemical bonds, so when chemical bonds are broken, energy is released. Cells take advantage of the fact that chemical bonds store energy. They purposefully break bonds to use the released energy. 3. Energy conversions: energy is converted between forms. When you pull the rubber band back, you are converting kinetic energy (moving your hand & the rubber band) to stored energy. Whenever energy is converted between forms, some of the energy is converted to heat, whether you mean to do that or not. So, when cells break chemical bonds to release energy to do work, some heat is always produced. Homeotherms (warm-blooded animals) take advantage of that fact to maintain stable temperatures. We "waste" tons of energy, just contracting & releasing muscles constantly to break bonds & release heat. Your pet boa constrictor can go without food for much longer than your pet guinea pig because the boa wastes much less energy on heat production. 4. Ways that cells store energy: keep this in mind, we will look at three specific modes of energy storage used by cells. Come back here and fill it in! B. Types of reactions 1. Decomposition/catabolism- breaking molecules down into smaller components. Overall, tends to release energy. Hydrolysis is a type of decomposition reaction, where water is split into H and OH, which are used to stabilize the 2 resulting molecules. Cells break down glucose to release it's energy. The cell then repackages that energy in smaller, portable ATP. When this happens, glucose (C6H12O6) is split into water (H20) and carbon dioxide (CO2). 2. Synthesis-building larger molecules from smaller components. Overall, tends to consume energy. Dehydration synthesis (condensation) is a type of synthesis reaction where one molecule loses a H, the other loses a OH, and water is formed. C. Enzymes- Increase the rate of reactions by lowering the activation energy, or energy required to get a reaction going. Without enzymes, the reactions in our cells wouldn't happen fast enough to sustain life. Each cell makes the enzymes it needs for the reactions it needs to carry out. III. Inorganic compounds Go over in book: properties of water, aqueous solutions, H+ in body fluids, pH, acids, bases, salts & buffers. Here's an explanation of acids and buffers: First, a little bit about water and pH. Water, as you know, is composed of two hydrogen atoms (H) bound to one oxygen atom (O): H2O. A glass of water contains millions of molecules of H2O. Being a liquid, these molecules are constantly moving around, bumping into the sides of the glass and each other. Occasionally, collisions cause a molecule to dissociate, or break up. When this happens, the H2O molecule is broken into a hydrogen ion (H+, which is called a proton for convenience) and a hydroxide (OH-). The hydroxide takes an electron (negative charge) from the hydrogen, which is why they end up with charge. Now we say these protons and hydroxides are "free" in solution, which means they are floating around on their own, not bound to any other particles. In pure water, the number of free H+ must equal the number of free OH-, because the dissociation of H2O always produces one of each. In this situation, when free H+ equals free OH-, the solution is neutral. A neutral solution has a pH of 7, which is a way of describing the concentration of free H+ relative to the concentration of free OH-. If we could drop extra H+ into the water without adding extra OH-, we would end up with an acid solution. Acid solutions have pH ranging from 0-7. Stronger acids have a low pH (for example, vinegar or acetic acid is around pH 5, while the stronger stomach or hydrochloric acid is around pH 2). If we could drop extra OH- into solution without adding H+, we would end up with a basic solution, pH 7-14, with 14 being the strongest. The remainder of the explanation will focus on acids since they are what we will encounter most with living systems. Acids are compounds that release H+ when dropped in water. So, if you took a bunch of dry HCl (hydrochloric acid) molecules, they'd probably look like a powder, and they'd be bound together as HCl. When you drop those molecules in water, however, most of them would immediately dissociate into H+ and Cl- (chloride), completely separate, not really giving a hoo-ha about what the other is doing. Strong acids are compounds that tend to dissociate immediately and almost completely. HCl is a strong acid, so when dropped in water, practically all of the Climmediately drop their H+ s like a hot potato. What this means is that strong acids throw LOTS of H+ into solution. H+ tends to react with other stuff, which gives acidic solutions their properties. Weak acids are compounds that tend to dissociate a little bit. H2CO3 (carbonic acid) dissociates into H+ and HCO3(bicarbonate) in water. Carbonic acid is a weak acid, so when dropped in water, only some of the HCO3- drop their H+. In addition, the other HCO3- are still on the fence about H+; they will take them back sometimes to become H2CO3 again. The H+ and anion of a weak acid kind of have an "on again, off again" relationship. The anions of a weak acid will bind to H+ again, if there are a lot of H+ around. Buffers are generally weak acids. Buffers can maintain a solution within a range of pH. That's because the anions of weak acids will drop their H+ if there aren't many H+ in solution, but will bind with H+ if there are lots of H+ in solution. To say that another way, when H+ concentrations rise, buffers bind them and take them out of solution. When H+ concentrations fall, buffers release them and put them back into solution. Buffers, therefore, keep the number of free H+ relatively constant. Since it is the concentration of free H+ in solution that determine acidity, buffers keep pH relatively constant. IV. Organic Compounds A. Functional groups: hydroxide, carboxyl, amino, phosphate. Carboxyls tend to lose H+ in water. Amino groups can pick up an extra H+ (-NH3) if H+ concentration is high. B. Carohydrates- typically end in "-ose" 1. Monosaccharides- The major ones we will consider all have the formula C6H12O6. Tend to form hexagon ("hexose") or pentagon ("pentose") rings, and you will see them illustrated in this way. The root "gly" refers to glucose (ex. proteoglycans = protein linked to polysaccharide). Glucose is the primary monosaccharide we will consider, but we will also talk about fructose and galactose when we get to the digestive system. 2. Di- and polysaccharides- two or many monosaccharides linked in branched chains. For example, the disaccharide sucrose (table sugar) is composed of one glucose attached to one fructose. Starch and many fibers are polysaccharides composed of thousands of glucose attached in chains (like a pearl necklace). We will talk a lot about the polysaccharide glycogen, which is also made of chains of glucose. Animals store glucose in certain cells with glycogen. Reference the pictures above under "types of reactions" to see examples of mono- and disaccharides. C. Lipids- nonpolar, hydrophobic, lipophilic 1. Fatty acids a. Glycerides: storage of fatty acids b. Phospholipids: using fatty acids as the fat-soluble end of an amphipathic molecule 2. Eicosanoids- Used by cells to send LOCAL chemical messages. Kind of a folded, modified fatty acid. 3. Sterols D. Proteins- root "pep" refers to proteins/bonded amino acids 1. Long folded, convoluted chains of amino acids; R-group determines amino acid; often carry an overall negative charge (because of many of the carboxyls losing their H+, plus many of the R groups tend to lose H+). 2. Shape and function -four levels, H-bonding and disulfide bonds, denaturation 3. Enzymes- function, action, saturation, factors that affect rate of activity. E. Nucleic Acids- DNA and RNA F. High Energy Compounds: Cells use the energy from foods to do work. Glucose, fatty acids, and amino acids can all be used by cells for energy; that is, cells can break the bonds in those molecules and use the energy released. Cells do not use that released energy directly. Instead, they carefully move the energy stored in "food" bonds to energy stored in the bonds of ATP. Many ATPs can be produced from one glucose, fatty acid or amino acid. ATP is composed of one adenosine (an organic molecule) and 3 phosphates (an inorganic side group, abbreviated Pi). When a cell needs energy to do work, it breaks the bonds between the 2nd or 3rd phosphate of ATP, and uses the energy released to do work directly. ATP is considered a "high energy compound," because it stores energy that can be easily accessed. It is not the only high-energy compound. We will talk about others when their contributions are important to specific systems. When a cell uses "food" energy to make ATP, it uses the energy released from glucose, fatty acids or amino acids to add phosphate/s to AMP (Adenosine MonoPhosphate) or ADP (Adenosine DiPhosphate). ATP is like the cell’s AA battery; it is portable, it has just the right amount of energy to do one job, AND it is rechargeable. When ATP is used to do a job, it splits into ADP +Pi. The cell can then recharge it by extracting some energy from glucose or fatty acids, and using that energy to re-attach the phosphate. Viola, now the cell can do another job!