Overview of the Human Body Chapter 1
advertisement

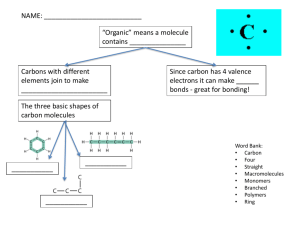
Overview of the Human Body Chapter 1 Overview • • • • Anatomy and physiology: definitions Form and Function Homeostasis Levels of organization What is “Anatomy and Physiology”? • Anatomy- • Physiology- Form (Structure) Fits Function • Principle of Complementarity- • You need to understand the parts and how they are put together before you can truly understand how they work • If you know what a body structure does, you can usually predict how it is organized/structured to do its job Homeostasis • Homeostasis- • The nervous system and endocrine system (hormones) play important roles in communication that promotes homeostasis throughout the body. • Every cell of the body must also maintain homeostasis Homeostasis and Feedback Loops • All systems involve three components: a ‘receptor’, a control center and an effector Stimulus (input into the system) RECEPTOR (ie. free nerve ending in the skin) CONTROL CENTER (such as the brain) EFFECTOR (such as a muscle, or a gland) The response to the stimulus leads to change. The change is ‘fed back’ to the receptor. Response (system’s output) Homeostasis and Feedback Loops • In negative feedback a stimulus causes a response which works to reduce/counteract the stimulus – Examples? • In positive feedback a stimulus causes a response which further increases the stimulus, so that output is accelerated – Examples? Levels of Organization • How big is an atom? ~ 10,000,000 atoms lined up side by side to measure 1 mm Atoms Molecule Organelle Smooth muscle cell 2 Cellular level 1 Chemical level Smooth muscle tissue Heart Blood vessels 3 Tissue level Tissues consist of similar types of cells. Blood vessel (organ) Smooth muscle tissue Connective tissue Epithelial tissue 4 Organ level Organs are made up of different types of tissues. 6 Organismal level 5 Organ system level Chemistry Chapter 2 Chemistry Basics • Element: unique substance that cannot be broken down into a more simple substance by ordinary chemical methods. Chemistry Basics • Atom: building block of an element – Proton – Neutron – Electron • Atomic Number • Atomic Mass Chemistry Basics • Isotope: different # of neutrons (from a standard atom of the same element) • Ion: different # of electrons (from the standard atom of the same element) Chemical Bonds • Chemical bonds: energy relationships between electrons of reacting atoms • Electrons in valence shell (outermost electron shell) – chemically reactive electrons • Octet rule (rule of eights) – Except for the first shell (full with two electrons) atoms interact to have eight electrons in their valence shell • 3 major bond types – Ionic – Covalent – Hydrogen Ionic Bonds + Sodium atom (Na) (11p+; 12n0; 11e–) Chlorine atom (Cl) (17p+; 18n0; 17e–) Sodium gains stability by losing one electron, and chlorine becomes stable by gaining one electron. Figure 2.6a–b Formation of an ionic bond. Sodium ion (Na+) — Chloride ion (Cl–) Sodium chloride (NaCl) After electron transfer, the oppositely charged ions formed attract each other. Covalent Bonds Reacting atoms Resulting molecules + or Structural formula shows single bonds. Carbon atom Hydrogen atoms Formation of four single covalent bonds: Carbon shares four electron pairs with four hydrogen atoms. Figure 2.7a Formation of covalent bonds. Molecule of methane gas (CH4) Types of Covalent Bonds • Non-Polar: Electrons shared equally – Produces electrically balanced, nonpolar molecules • Polar: unequal sharing of electrons produces polar – Atoms in bond have different electron-attracting abilities Electrons and chemical bonds H2 H2O •Which molecule above is polar? Non-polar? How do you know? •Which molecule above has covalent bonds? Polar covalent bonds? •Are either of these molecules ions? Other important terms • Hydrophilic• Hydrophobic- A triglyceride H 2O pH- Acids and Bases • Acids – Have a sour taste – Release hydrogen ions, protons (H+) into solution – HCl, HC2H3O2, H2CO3 • Bases – Have a bitter taste, feel slippery – Are proton acceptors (they take up H+s from solution) – NaOH, HCO3-, NH3 pH- Acid-Base Concentration • The concentration of H+ ions in solution is measured in units of pH • The pH scale is logarithmic and runs from 0 to 14, with a pH value of 7 indicating a neutral solution – Acidic solutions have pH values from 0-6 – Neutral solutions = pH 7 – Basic (alkaline) solutions have pH values from 8-14 • The more hydrogen ions in a solution, the more acidic it is, but the lower its pH value. Thought Question • The presence of hydrogen ions stimulates the brain to increase respiration rate. • Johnny’s blood pH is 7.25. Normal blood pH is 7.4. • Will Johnny likely be breathing faster or slower than normal? Why? Chemical Reactions 1. Synthesis or Anabolic reactions 2. Decomposition or Catabolic reactions 3. Exchange reactions (swapping partners) Chemical Reactions • Synthesis or Anabolic reactions A + B → AB • Decomposition/Catabolic Reactions AB → A + B • Exchange reactions AB + CD → AD + CB What type of reaction is this? Sucrose Glucose Glucose O Fructose Fructose Reactant A. B. C. D. & Fructose Fructose Products Synthesis Reaction Decomposition Reaction Exchange Reaction All of the above Chemical Reactions, Energy & Enzymes • Most chemical reactions do not occur spontaneously, or they occur so slowly that they would be of little value to cells • Activation energy- • Enzymes promote chemical reactions by lowering activation energy • Enzymes are biological catalysts, they are usually protein molecules Mechanism of enzyme action Substrates (S) e.g., amino acids + Product (P) e.g., dipeptide Energy is absorbed; bond is formed. Water is released. Peptide bond Active site Enzyme (E) Enzyme-substrate complex (E-S) 1 Substrates bind 2 Internal at active site. rearrangements Enzyme changes leading to shape to hold catalysis substrates in occur. proper position. Link- Enzyme animation Enzyme (E) 3 Product is released. Enzyme returns to original shape and is available to catalyze another reaction. Sucrase is the enzyme that catalyzes this decomposition reaction Sucrose Glucose sucrase Glucose O Reactant Fructose Fructose & Fructose Fructose Products What do you think? -Does the reverse reaction ever occur? -Is sucrose ever formed (synthesized) from glucose and fructose? What are organic compounds? • In chemistry, an organic compound must contain carbon and hydrogen • Most biologically relevant, organic compounds are soluble in water – Why? – What group might be the exception? • Many are polymers (large molecules) built from monomers (small subunits) What are examples of inorganic compounds in the body? What are some examples of these organic compounds in the body? 1. 2. 3. 4. Carbohydrates Lipids Proteins Nucleic acids Can you identify an example of each? Carbohydrates • Group of molecules that includes sugars, starches and fiber • Account for less then 1-2% of body weight • In the body, primary function is as a readily usable energy source (glucose) – Also as energy storage (glycogen) – Cellular surface markers • Forms of carbohydrates – Monosaccharides, Disaccharides, Polysaccharides Glucose is a monosaccharide C6H12O6 = = = Fuel glucose • Glucose is a ‘single’ sugar. • Note that it is a ring structure with 6 carbon atoms. • Other monosaccharides- fructose, galactose Sucrose is a disaccharide Glucose O Fructose Fructose Other disaccharides- maltose, lactose Polysaccharides • Starch/Amylose • Glycogen • Cellulose Linear chain Polysaccharides Glucose monomer Starch granules in potato tuber cells (a) Starch Glycogen Granules In muscle tissue (b) Glycogen Cellulose fibril in a plant cell wall Cellulose molecules (c) Cellulose Monomer + Monomer + Monomer = Polymer Lipids • Lipids, as a class, are a very diverse group of molecules – What do you think is the unifying characteristic of lipids? • Lipids are important energy stores • Lipids form essential structures in cells Major types of lipids 1. Triglycerides - Comprised of fatty acids and glycerol; what we usually call ‘fats’ or ‘oils’ 2. Steroids -Cholesterol derivatives 3. Eicosanoids -Cell signaling molecules 4. Phosopholipids - Amphipathic molecules that form cell membranes Triglycerides FATTY ACID FATTY ACID FATTY ACID • Triglycerides are three fatty acids linked to one glycerol molecule. • Fatty acids are long, linear chains of carbon and hydrogens (hydrocarbon chains). Triglycerides FATTY ACID #1 • In different triglycerides, the glycerol is the same, but the fatty acid chains vary, resulting in different types of fats and oils. • All fatty acid (hydrocarbon chains) are non-polar. Functions and locations of triglycerides in the body • Functions of triglycerides? 1. FATTY ACID 2. FATTY ACID 3. FATTY ACID • Where can we find stores of triglycerides? Steroids • All consist of a complex ring structure • Cholesterol is the precursor for all steroid hormones – Estrogen – Testosterone – Cortisol cholesterol • Signaling molecules – Sexual function – Tissue metabolism • Component of animal cell membranes estrogen testosterone Eicosanoids • Diverse group of lipids derived from fatty acids of cell membranes – Prostaglandins • Powerful signaling molecules, synthesized by nearly all tissues of the body • Tend to act locally • Pain/inflammation • Labor Cell • Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) inhibit prostaglandin synthesis Cell membranae Phospholipids Phospholipids are modified triglycerides. Phospholipids have TWO fatty acids chains, and a phospho-group in place of the third! A triglyceride Non-lipid Group Phosphate Group A phospholipid = Phospholipid Chemist’s version Anatomist/Biologist’s version Phospholipid molecules are amphipathic. One part of the molecule is polar, while the other end is non-polar. Which end interacts readily with water? Phospholipid bilayer Chemist’s version Biologist’s version H20 Sesame Street version Two layers of phospholipids, stacked on each other, with the hydrophobic tails of each layer facing one another. Phospholipid animation http://telstar.ote.cmu.edu/biology/MembranePage/index2.html All cell membranes are a phosophlipid bilayer • Forms the boundary between a cell and its environment • Hydrophobic core, with hydrophilic ends Proteins • Proteins are polymers (chains) of amino acids (AA) • A single, generalized amino acid Heidi’s Protein CartoonEach shape in the chain represents one amino acid Peptide bonds - + - + •Amino acids are linked together via peptide bonds. •Two linked amino acids = dipeptide •10-50 linked together = polypeptide •50+ = protein Protein Functions The over 2 million proteins in our bodies do an amazing variety of tasks • • • • • • • • Enzymes (control metabolism) Support Movement Transport Cell Receptors for communication Buffering Hormonal Regulation Defense The 20 Amino Acids Protein Structure The hydrogen bonds between amino acids (not the peptide bonds) are easily disrupted by changes in temperature and pH. What happens to protein structure when pH is abnormal? LINK Nucleic acids • DNA- deoxyribonucelic acid and RNA- ribonucleic acid -Store and process information -Your genetic code -Provide the directions for building proteins • DNA is a double stranded molecule that resides in the cell nucleus • RNA is single stranded molecule that is found mainly outside of the nucleus, usually serving as a ‘copy’ of DNA Nucleic Acids are Polymers, too! • The monomers/units of nucleic acids are nucleotides • A nucleotide consists of – Sugar – Phosphate group – Nitrogenous base (A, C, T, G or U) Phosphate Base Sugar Nucleotide monomer DNA and RNA •DNA is two long strands of nucleotides, held together by hydrogen bonds between the nucleotide bases -Exhibits complementary base pairing along DNA double helix •RNA is a single stranded nucleic acid; serves as a template for protein synthesis RNA Adenosine Triphosphate- ATP • Storable “energy packets” for cells - - - Adenosine Link http://faculty.ccbcmd.edu/biotutorials/energy/adpan.html Cellular work driven by ATP energy Solute + • Hydrolysis of ATP provides energy for cellular needs • ATP transfers its terminal phosphate, and ‘energizes’ another molecule Membrane protein (a) Transport work: ATP phosphorylates transport proteins, activating them to transport solutes (ions, for example) across cell membranes. + Relaxed smooth muscle cell Contracted smooth muscle cell (b) Mechanical work: ATP phosphorylates contractile proteins in muscle cells so the cells can shorten. + (c) Chemical work: ATP phosphorylates key reactants, providing energy to drive energy-absorbing chemical reactions.