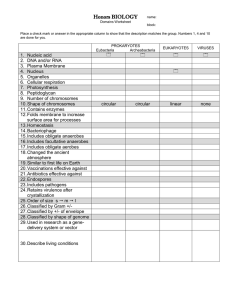
Microbial Growth • Physical Requirements of Microbes
advertisement
Microbial Growth • Physical Requirements of Microbes • Temperature (optimal enzyme operation) • • • • pH (optimal enzyme operation) • Using buffers in media • Molds & yeasts versus bacteria Chemical Requirements • Carbon source in medium • Nitrogen, sulfur, phosphorous, trace elements • Oxygen requirements • Obligate aerobes, anaerobes, facultative anaerobes • Free radical oxygen (O2-) and H2O2 dangers; superoxide dismutase and catalase = aerobes Culture Media for Microbes • Chemically defined vs. complex media • Anaerobes: reducing media/Brewer jar • Other: animals, eggs, tissue culture, CO2 • Media types • • Psychrophiles, mesophiles, thermophiles Selective, Differential, Enrichment Bacterial Population Growth • Growth Curve: Lag, Log, Stationary, Death • Quantifying Growth Characterizing Microbes By Optimal Growth Temperature Figure 6.1 Temperature Growth Ranges and Food Safety “2-40-140” If > 2 hrs at 40-140oF, don’t eat it! Figure 6.2 Alkalophiles Neutrophiles Acidophiles Physical Requirements: pH • Most bacteria grow between pH 6.5 and 7.5 • Molds and yeasts grow between pH 5 and 6 Physical Requirements: Osmotic Pressure • Hypertonic environments, increase salt or sugar, cause plasmolysis • Extreme or obligate halophiles require high osmotic pressure • Facultative halophiles tolerate high osmotic pressure Microbial Growth • Physical Requirements of Microbes • Temperature (optimal enzyme operation) • • • pH (optimal enzyme operation) • Using buffers in media • Molds & yeasts versus bacteria Chemical Requirements • Carbon source in medium • Nitrogen, sulfur, phosphorous, trace elements • Oxygen requirements • • • Obligate aerobes, anaerobes, facultative anaerobes Free radical oxygen (O2-) and H2O2 dangers; superoxide dismutase and catalase = aerobes Culture Media for Microbes • Chemically defined vs. complex media • Anaerobes: reducing media/Brewer jar • Other: animals, eggs, tissue culture, CO2 • Media types • • Psychrophiles, mesophiles, thermophiles Selective, Differential, Enrichment Bacterial Population Growth • Growth Curve: Lag, Log, Stationary, Death • Quantifying Growth The Requirements for Growth: Chemical Requirements • Carbon • Structural organic molecules, energy source • Chemoheterotrophs use organic carbon sources • Autotrophs use CO2 The Requirements for Growth: Chemical Requirements • Nitrogen • In amino acids, proteins • Most bacteria decompose proteins • Some bacteria use NH4+ or NO3 • A few bacteria use N2 in nitrogen fixation • Sulfur • In amino acids, thiamine, biotin • Most bacteria decompose proteins • Some bacteria use SO42 or H2S • Phosphorus • In DNA, RNA, ATP, and membranes • PO43 is a source of phosphorus • Trace Elements • Inorganic elements required in small amounts • Usually as enzyme cofactors Microbial Growth • Physical Requirements of Microbes • Temperature (optimal enzyme operation) • • • • pH (optimal enzyme operation) • Using buffers in media • Molds & yeasts versus bacteria Chemical Requirements • Carbon source in medium • Nitrogen, sulfur, phosphorous, trace elements • Oxygen requirements • Obligate aerobes, anaerobes, facultative anaerobes • Free radical oxygen (O2-) and H2O2 dangers; superoxide dismutase and catalase = aerobes Culture Media for Microbes • Chemically defined vs. complex media • Anaerobes: reducing media/Brewer jar • Other: animals, eggs, tissue culture, CO2 • Media types • • Psychrophiles, mesophiles, thermophiles Selective, Differential, Enrichment Bacterial Population Growth • Growth Curve: Lag, Log, Stationary, Death • Quantifying Growth How Toxic Forms of Oxygen Are Handled • Singlet oxygen: O2 boosted to a higher-energy state • Handling superoxide free radicals: O2 2O2- + 2O2- + 8H+ oxygen radicals • 4H2O2 hydrogen peroxide Superoxide Dismutase (SODS) • Handling peroxide anion: O22 2H2O2 hydrogen peroxide 2H2O + water oxygen gas Catalase (Peroxidase) Catalase Test: Bacteria + H2O2 bubbles O2 Obligate aerobes Faultative anaerobes Obligate anaerobes Aerotolerant anaerobes Microaerophiles Thyoglycollate binds molecular oxygen, reducing it and removing it: R-SH + O2 R-SO2 Microbial Growth • Physical Requirements of Microbes • Temperature (optimal enzyme operation) • • • • pH (optimal enzyme operation) • Using buffers in media • Molds & yeasts versus bacteria Chemical Requirements • Carbon source in medium • Nitrogen, sulfur, phosphorous, trace elements • Oxygen requirements • Obligate aerobes, anaerobes, facultative anaerobes • Free radical oxygen (O2-) and H2O2 dangers; superoxide dismutase and catalase = aerobes Culture Media for Microbes • Chemically defined vs. complex media • Anaerobes: reducing media/Brewer jar • Other: animals, eggs, tissue culture, CO2 • Media types • • Psychrophiles, mesophiles, thermophiles Selective, Differential, Enrichment Bacterial Population Growth • Growth Curve: Lag, Log, Stationary, Death • Quantifying Growth Culture Media: Chemically Defined or Complex Table 6.2 & 6.4 Anaerobic and Low O2 Culture Methods Candle jar Brewer or anaerobic jar CO2 packet Unusual Culture Methods Grows only in certain cell types: using armadillos to culture M. leprae Grows only inside live cells: eggs as culture vessels for influenza virus Grows only in certain cell types: using tissue culture with low O2, enriched CO2 incubators Microbial Growth • Physical Requirements of Microbes • Temperature (optimal enzyme operation) • • • • pH (optimal enzyme operation) • Using buffers in media • Molds & yeasts versus bacteria Chemical Requirements • Carbon source in medium • Nitrogen, sulfur, phosphorous, trace elements • Oxygen requirements • Obligate aerobes, anaerobes, facultative anaerobes • Free radical oxygen (O2-) and H2O2 dangers; superoxide dismutase and catalase = aerobes Culture Media for Microbes • Chemically defined vs. complex media • Anaerobes: reducing media/Brewer jar • Other: animals, eggs, tissue culture, CO2 • Media types • • Psychrophiles, mesophiles, thermophiles Selective, Differential, Enrichment Bacterial Population Growth • Growth Curve: Lag, Log, Stationary, Death • Quantifying Growth Selective Media • Goal: To chemically (or physically) suppress unwanted microbes and encourage desired microbes. MSA Mannitol salt agar : selective for halophiles with 7% salt (osmotic challenge) and differential for mannitol fermenters: good for skin bacterial cultures. EMB Agar: kills gram positives with eosin and methylene blue, selective for gram negatives. Differential for lactose fermenters. Good for growing enterics. McConkey Agar: supresses gram positives with crystal violet and bile salts; also differential for EMB MA Figure 6.9b, c Differential Media • Distinguish between different species based on a metabolic ability. Blood agar (sheep’s blood) reveals if hemolytic Mannitol salt agar contains the pH sensitive dye phenol red (yellow when acidic) Se Sa Figure 6.9a Enrichment Media • Encourages growth of desired microbe by providing special growth conditions or added growth factors Thioglycollate Anaerobic or Brewer Jar Lysed red blood cells provide unique nutrients in blood/chocolate agar Glucose Salts Agar (enriches for microbes that can growth only on glucose and some inorganic nutrients Pure Cultures Used To Study Characteristics Of A Particular Species • A pure culture contains only one species or strain • A colony is a population of cells arising from a single cell or spore or from a group of attached cells • A colony is often called a colony-forming unit (CFU) Microbial Growth • Physical Requirements of Microbes • Temperature (optimal enzyme operation) • • • • pH (optimal enzyme operation) • Using buffers in media • Molds & yeasts versus bacteria Chemical Requirements • Carbon source in medium • Nitrogen, sulfur, phosphorous, trace elements • Oxygen requirements • Obligate aerobes, anaerobes, facultative anaerobes • Free radical oxygen (O2-) and H2O2 dangers; superoxide dismutase and catalase = aerobes Culture Media for Microbes • Chemically defined vs. complex media • Anaerobes: reducing media/Brewer jar • Other: animals, eggs, tissue culture, CO2 • Media types • • Psychrophiles, mesophiles, thermophiles Selective, Differential, Enrichment Bacterial Population Growth • Growth Curve: Lag, Log, Stationary, Death • Quantifying Growth Bacterial Growth is Exponential (Logarithmic) Bacterial “growth” means an increase in the number of individuals, not an increase in cell size. Figure 6.12b Growth Curve for Bacteria (Logarithmic Plot) Figure 6.14 Estimating Bacterial Numbers by Indirect methods • Direct Measures • Plate counts of viable bacterial forming colonies • Counting low viable bacterial numbers by filtration • Counting viable bacteria with Most Probable Number • Counting bacteria per ml in direct microscopy • Indirect Measures • Turbidity/Absorbance with a spectrophotometer • Metabolic activity tracking conversion of colored molecules • Dry weight by weighing a set volume and knowing weight of one cell Plate Assays: Spread Plate or Pour Plate Methods • After incubation, count colonies on plates that have 30300 colonies (CFUs) The dilution in a particular tube = ml of fluid added to tube/total volume after addition; e.g. 1ml/(9ml + 1ml) = 1/10 = 10-2 Figure 6.15 Direct Measurements of Microbial Growth Figure 6.19 Direct Measurements of Microbial Growth • Filtration: Good for measuring very dilute samples of bacteria Figure 6.17a, b Direct Measurements of Microbial Growth • Multiple tube MPN test • Count positive tubes and compare to statistical MPN table • Produces a range of concentrations Figure 6.18b Estimating Bacterial Numbers by Indirect methods • Direct Measures • Plate counts of viable bacterial forming colonies • Counting low viable bacterial numbers by filtration • Counting viable bacteria with Most Probable Number • Counting bacteria per ml in direct microscopy • Indirect Measures • Turbidity/Absorbance with a spectrophotometer • Metabolic activity tracking conversion of colored molecules/enyzme assay • Dry weight by weighing a set volume and knowing weight of one cell Estimating Bacterial Numbers by Indirect Methods • Turbidity Figure 620 Metabolic Conversion/Enzyme Assay • 1 bacterium produces 4.6 x 1012 NADH/sec/cell under idea growth conditions. • In a 1 ml sample of growing cells, 5.2 x 1023 NADH/sec/ml are produced per second (as revealed by a color-based assay of NADH on the sample) • Therefore, (4.6 x 1012 NADH/sec/ml) x (5.2 x 1023 NADH/sec/cell) = 2.3 x 1024 cells/ml Determining dry mass of a fixed volume An E. coli cell has a dry mass of about 7.0 x 10-19 mg. A 1 ml sample with a dry mass of 2 mg therefore has: 2 mg/ml x 1 cell/7 x 10-19 mg = 2.8 x 1020 cells/ml Microbial Growth • Physical Requirements of Microbes • Temperature (optimal enzyme operation) • • • • pH (optimal enzyme operation) • Using buffers in media • Molds & yeasts versus bacteria Chemical Requirements • Carbon source in medium • Nitrogen, sulfur, phosphorous, trace elements • Oxygen requirements • Obligate aerobes, anaerobes, facultative anaerobes • Free radical oxygen (O2-) and H2O2 dangers; superoxide dismutase and catalase = aerobes Culture Media for Microbes • Chemically defined vs. complex media • Anaerobes: reducing media/Brewer jar • Other: animals, eggs, tissue culture, CO2 • Media types • • Psychrophiles, mesophiles, thermophiles Selective, Differential, Enrichment Bacterial Population Growth • Growth Curve: Lag, Log, Stationary, Death • Quantifying Growth