Followings are what you will find at the end of... For LEARNING OBJECTIVES: Highlight the main idea for EACH...
advertisement

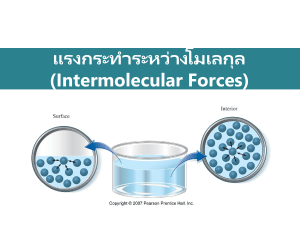
Pre CH12 HW for Silberberg Followings are what you will find at the end of the chapter in your textbook. For LEARNING OBJECTIVES: Highlight the main idea for EACH objective. Ready carefully so you don’t highlight everything. For MASTER THESE SKILLS: Highlight the main idea for each skill. Ready carefully so you don’t highlight everything. For KEY TERMS: Make sure you can define it and/or give an example of it. Pick TWO terms of your choice and actually write the definition or an example. For KEY EQUATIONS AND RELATIONSHIP: Next to EACH, define each term. Be very specific. Page 507 CHAPTER REVIEW GUIDE Learning Objectives Relevant section (§) and/or sample problem (SP) numbers appear in parentheses. Understand These Concepts 1. How the interplay between kinetic and potential energy underlies the properties of the three states of matter and their phase changes (§12.1) 2. The processes involved, both within a phase and during a phase change, when heat is added or removed from a pure substance (§12.2) 3. The meaning of vapor pressure and how phase changes are dynamic equilibrium processes (§12.2) 4. How temperature and intermolecular forces influence vapor pressure (§12.2) 5. The relation between vapor pressure and boiling point (§12.2) 6. How a phase diagram shows the phases of a substance at differing conditions of pressure and temperature (§12.2) 7. The distinction between bonding and intermolecular forces on the basis of Coulomb's law and the meaning of the van der Waals radius of an atom (§12.3) 8. The types and relative strengths of intermolecular forces acting in a substance (dipole-dipole, H bonding, dispersion), the impact of H bonding on physical properties, and the meaning of polarizability (§12.3) 9. The meanings of surface tension, capillarity, and viscosity and how intermolecular forces influence their magnitudes (§12.4) 10. How the important macroscopic properties of water arise from atomic and molecular properties (§12.5) 11. The meaning of crystal lattice and the characteristics of the three types of cubic unit cells (§12.6) 12. How packing of spheres gives rise to the hexagonal and cubic unit cells (§12.6) 13. Types of crystalline solids and how their intermolecular forces give rise to their properties (§12.6) 14. How band theory accounts for the properties of metals and the relative conductivities of metals, nonmetals, and metalloids (§12.6) 15. The structures, properties, and functions of modern materials (doped semiconductors, liquid crystals, ceramics, polymers, and nanostructures) (§12.7) Master These Skills 1. Calculating the overall enthalpy change when heat is gained or lost by a pure substance (§12.2 and SP 12.1) 2. Using the Clausius-Clapeyron equation to examine the relationship between vapor pressure and temperature (SP 12.2) 3. Using a phase diagram to predict the physical state and/or phase change of a substance (§12.2 and SP 12.3) 4. Determining whether a substance can form H bonds and drawing the H-bonded structures (SP 12.4) 5. Predicting the types and relative strength of the bonding and intermolecular forces acting within a substance from its structure (SP 12.5) 6. Finding the number of particles in a unit cell and the coordination number (§12.6 and SP 12.6) 7. Calculating atomic radius or unit-cell edge length from the crystal structure of an element (SPs 12.7, 12.8) Key Terms Page numbers appear in parentheses. Section 12.1 phase (455) intermolecular forces (455) phase change (456) condensation (457) = a phase change when (g) (l) vaporization (457) freezing (457) melting (fusion) (457) sublimation (457) deposition (457) heat of vaporization ( heat of fusion ( ) (457) ) (457) heat of sublimation ( ) (458) Section 12.2 heating-cooling curve (459) vapor pressure (463) Clausius-Clapeyron equation (464) boiling point (465) melting point (465) phase diagram (466) triple point (466) critical point (466) Section 12.3 van der Waals radius (468) ion-dipole force (469) dipole-dipole force (469) hydrogen bond (H bond) (470) polarizability (472) dispersion (London) force (472) Section 12.4 surface tension (475) capillarity (475) viscosity (476) Section 12.6 crystalline solid (479) amorphous solid (479) lattice (479) unit cell (479) coordination number (480) simple cubic unit cell (480) body-centered cubic unit cell (480) face-centered cubic unit cell (480) packing efficiency (482) hexagonal closest packing (482) cubic closest packing (482) x-ray diffraction analysis (486) scanning tunneling microscopy (486) atomic solid (488) molecular solid (488) ionic solid (488) metallic solid (489) network covalent solid (489) band theory (490) valence band (491) conduction band (491) conductor (492) semiconductor (492) insulator (492) superconductivity (492) Section 12.7 crystal defect (493) doping (494) liquid crystal (495) ceramic (498) polymer (500) macromolecule (500) monomer (500) degree of polymerization (n) (500) random coil (501) radius of gyration (Rg) (501) plastic (503) branch (503) crosslink (504) elastomer (504) copolymer (504) nanotechnology (505) Page 508 Key Equations and Relationships Page numbers appear in parentheses. 12.1 Using the vapor pressure at one temperature to find the vapor pressure at another temperature (twopoint form of the Clausius-Clapeyron equation) (464): P1 = vapor pressure at T1 P2 = vapor pressure at T2 Hvap = heat of vaporization in J/mol R = 8.314J/Kmol Ti and T2 = Temp in K Answer the following questions. Show your work. (a) Is CO2 molecule polar? Explain briefly. (b) List all types of solids (atomic, molecular, ionic…) and give one example each. (c) Sketch three cubic unit cells. Name each. (d) Explain intermolecular forces in your own words. Give few examples of intermolecular forces. (e) Explain hydrogen bonding in your own words. (f) Sketch a Cooling Curve for water. Look up specific heat capacity of H2O(g), H2O(l), and H2)(s). Look up heat of fusion and heat of vaporization of water. Match these 5 values to the corresponding section on the cooling curve.