New Developments in Oral Anticoagulants: Treating and Preventing Embolic Events in the 21
advertisement

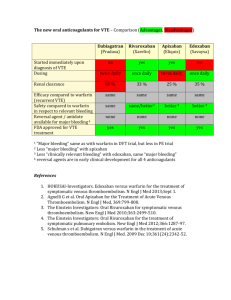
New Developments in Oral Anticoagulants: Treating and Preventing Embolic Events in the 21st Century David Stewart, PharmD, BCPS Assistant Professor of Pharmacy Practice East Tennessee State University Bill Gatton College of Pharmacy stewardw@etsu.edu Disclosures Speaker’s Bureau for: Boehringer-Ingelheim Pharmaceuticals Janssen Pharmaceuticals At the conclusion of this program, the audience should be able to: • List the new oral anticoagulant medications currently approved or in the approval process by the United States Food and Drug Administration • Communicate basic principles of pharmacokinetics to other healthcare providers • Identify appropriate indications for the use of new oral anticoagulant medications • Develop patient specific plans utilizing newly approved oral anticoagulant agents for the treatment and prevention of venous thromboembolic events in various patient populations Including warfarin, how many oral anticoagulants are currently FDA approved in the United States? One Two Three Four Five 0% Fiv e 0% Fo ur Tw 0% Th re e 0% o 0% On e 1. 2. 3. 4. 5. Warfarin Pros • Experience • Inexpensive • Reversible • Monitoring available Cons • Time of onset • Frequent monitoring • Dosing variability • Numerous drug interactions 21st Century • New therapies with FDA Approval – Dabigatran – Rivaroxaban • Additional emerging new therapies – Apixaban (Phase III trials complete) – Edoxaban (Phase III trials ongoing) Which of the following best describes your opinion regarding the novel new anticoagulant medications? 0% Ih av es er io he n ... us ... 0% Ip re fe rt li. . . 0% sti ll a ha fa rin I’m ... an is W ar fa rin 0% sm ... 0% W ar 1. Warfarin is an adequate medication with good data so I’ll continue to use warfarin. 2. Warfarin has many shortcomings and I would prefer to use newer agents. 3. I’m still a little hesitant about the newer agents because I’m unfamiliar with them. 4. I prefer the newer agents over warfarin; however, I am concerned about the cost of new agents. 5. I have serious concerns about the safety of newer anticoagulant medications. Which of the following best describes your current prescribing of dabigatran (Pradaxa®) or rivaroxaban (Xarelto®)? 1. I routinely prescribe them 2. I prescribe them in a limited and select group of patients 3. I am very hesitant to prescribe them 4. I have never prescribed them 0% ev er p. .. en Ih av ve ry he si. Ia m Ip re sc r ibe pr ... Ir ou t in e ly 0% .. 0% th ... 0% Clinical Pharmacology Comparison Coagulation Cascade Intrinsic Pathway (PTT) XII XIIa XI XIa IX VIIa VII IXa VIII Warfarin Rivaroxan & Apixaban Extrinsic Pathway (PT) XIII Xa Va X II IIa XIIIa Dabigatran Fibrinogen Fibrin Summary Table Parameter Apixaban Dabigatran Rivaroxaban Target Protein Factor Xa Thrombin (IIa) Factor Xa No Yes (etexilate) No CYP3A4/P-gp Renal CYP3A4/P-gp Avoid < 15 ml/min ↓ 15-30 ml/min Avoid < 15 ml/min Avoid < 30 ml/min CYP3A4/P-gp Rifampin (P-gp) CYP3A4/P-gp Onset of activity 3-4 hrs 1-2 hrs 2-4 hrs t½ 8-15 hrs 12-18 hrs 5-9 hrs Dosing interval Twice daily Twice daily Daily Measuring tests PT/Anti-factor Xa ECT, TT, +/- aPTT PT/Anti-factor Xa Pro-Drug 1˚ Elimination Renal Adjustment Drug-Drug Interact. Monitoring vs. Measuring Measuring Dabigatran Thromb Haemost 2010;103:1116-27. Measuring Rivaroxaban & Apixaban • Role of aPTT & PT/INR • Anti-Xa Assays – Chromagenic anti-Xa assays may be useful • Different assays vary in sensitivity • Must calibrate standard curve based on drug concentration – HepTest® is not accurate for rivaroxaban (and likely apixaban) • Incubation period too long • Modified HepTest® may be useful but no current data Ther Drug Monit 2010;32:673-9. Measuring Rivaroxaban PT is sensitive (Don’t rely on INR) aPTT not sensitive Highlights peak concentrations J Thromb Haemost 2011;9:133-9. Which of the following are significantly affected by moderate/severe renal insufficiency? Apixaban Dabigatran Rivaroxaban Both 2 & 3 All of the above 0% ab o. .. & Al l of t he 2 Bo th Ri va r ox a at ra 0% 3 0% ba n n 0% Da bi g xa b an 0% Ap i 1. 2. 3. 4. 5. Clinical Utilization What are the current approved indications for dabigatran (Pradaxa®) in the United States? Non-valvular Afib Prevention of VTE Treatment of VTE All of the above 0% Al l of t he ab o. .. 0% m en to fV . .. Tr ea t of ... 0% Pr ev en t io n al vu l ar A. .. 0% No nv 1. 2. 3. 4. Atrial Fibrillation Summary of Afib Data Apixaban (ARISTOTLE) Dabigatran (RE-LY) Rivaroxaban (ROCKET – AF) > 18,000 > 18,000 > 14,000 Mean CHADS2 ≈2 ≈2 ≈ 3.5 TTR 62% 64% 55% Superior Superior1 Non-Inferior Decreased Similar Similar # Patients Efficacy vs. VKA Bleeding2 vs. VKA 1Dabigatran 150 mg BID group. 2Major bleeding per study design. RE-LY - Results Event Dabi 110 vs Warf HR (95% CI) Dabi 150 vs Warf HR (95% CI) Dabi 150 vs Dabi 110 HR (95% CI) Efficacy 1˚ Endpoint* 0.91 (0.74-1.11) 0.66 (0.53-0.82) 0.73 (0.58-0.91) All stroke 0.92 (0.74-1.13) 0.64 (0.51-0.81) 0.70 (0.56-0.89) Ischemic Stroke 1.11 (0.89-1.40) 0.76 (0.60-0.98) 0.69 (0.54-0.88) Hemorrhagic Stroke 0.31 (0.17-0.56) 0.26 (0.14-0.49) 0.85 (0.39-1.83) MI published 1.35 (0.98-1.87) 1.38 (1.00-1.91) 1.02 (0.76-1.38) MI revised 1.29 (0.96-1.75) 1.27 (0.94-1.71) Not available All cause mortality 0.91 (0.80-1.03) 0.88 (0.77-1.00) 0.97 (0.85-1.11) Safety Major bleeding 0.80 (0.69-0.93) 0.93 (0.81-1.07) 1.16 (1.00-1.34) GI bleeding 1.10 (0.86-1.41) 1.50 (1.19-1.89) 1.36 (1.09-1.70) All bleeding 0.78 (0.74-0.83) 0.91 (0.86-0.97) 1.16 (1.09-1.23) IC bleeding 0.31 (0.20-0.47) 0.40 (0.27-0.60) 1.32 (0.80-2.17) New Engl J Med 2009;361:1139-51. New Engl J Med 2010;363:1875-76. *Non-inferiority margin = 1.46 RE-LY Summary • Dabigatran 110 mg BID vs. warfarin – Non-Inferior Efficacy – Lower major and overall bleeding rates – Similar GI bleeding rates • Dabigatran 150 mg BID vs. warfarin – – – – Superior efficacy Lower overall bleeding rates Similar major bleeding rates Elevated rates of GI bleeding • Both doses showed decreased ICH compared to warfarin (60-70% RRR) ROCKET-AF Results Event Rivaroxaban (% per year) Warfarin (% per year) HR (95% CI) Efficacy 1˚ Endpoint* 2.1 2.4 0.88 (0.75-1.03) All stroke 1.65 1.96 0.85 (0.70-1.03) Ischemic Stroke 1.34 1.42 0.94 (0.75-1.17) Hemorrhagic Stroke 0.26 0.44 0.59 (0.37-0.93) MI 0.91 1.12 0.81 (0.63-1.06) All-cause mortality 1.87 2.21 0.85 (0.70-1.02) Safety Major bleeding 3.6 3.4 1.04 (0.90-1.20) All bleeding 14.9 14.5 1.03 (0.96-1.11) 3.2 (% overall) 2.2 (% overall) p < 0.001 0.5 0.7 0.67 (0.47-0.93) Major GI bleeding IC bleeding *Non-inferiority margin = 1.46 New Engl J Med 2011;365:883-91. ROCKET-AF Summary • Rivaroxaban vs. warfarin – Non-Inferior Efficacy – Similar bleeding rates – Lower ICH rates • High risk patient population (Mean CHADS2 score > 3) • TTR 55% New Engl J Med 2011;365:883-91. ARISTOTLE Results Event Apixaban (% per year) Warfarin (% per year) HR (95% CI) Efficacy 1˚ Endpoint* 1.27 1.60 0.79 (0.66-0.95) All stroke 1.19 1.51 0.79 (0.65-0.95) Ischemic Stroke 0.97 1.05 0.92 (0.74-1.13) Hemorrhagic Stroke 0.24 0.47 0.51 (0.35-0.75) MI 0.53 0.61 0.88 (0.66-1.17) All-cause mortality 3.52 3.94 0.89 (0.80-0.998) Safety Major bleeding 4.07 6.01 0.68 (0.61-0.75) All bleeding 18.1 25.8 0.71 (0.68-0.75) IC bleeding 0.33 0.80 0.42 (0.30-0.58) *Non-inferiority margin = 1.38 New Engl J Med 2011;365:981-92. ARISTOTLE Summary • Apixaban vs. warfarin – – – – Superior efficacy with apixaban ↓ overall mortality with apixaban (NNT = 238) Lower bleeding rates with apixaban Lower ICH rates • Apixaban vs. Aspirin – – – – – 6,000 high-risk patients (mean CHADS2 = 2) Not candidates for warfarin 1 year follow-up Superior efficacy to aspirin Similar bleeding (including ICH) rates New Engl J Med 2011;365:981-92. New Engl J Med 2011;364:806-17. Which of the following have been shown at least as effective as warfarin for the prevention of stroke in patients with atrial fibrillation? 0% 0% ab o. .. he of t Al l Bo th 1 & ba n 2 0% ro xa at ra n 0% Da bi g xa b an 0% Ri va Apixaban Dabigatran Rivaroxaban Both 1 & 2 All of the above Ap i 1. 2. 3. 4. 5. Treatment of Acute Venous Thromboembolism Both dabigatran and rivaroxaban have been shown to be effective in the treatment of acute VTE. 1. True 2. False se 0% Fa l Tr ue 0% Summary of VTE Data1 Dabigatran (RE-COVER) Rivaroxaban (EINSTEIN) > 2,500 > 3,400 6 months 6 months LMWH Rivaroxaban 60% 58% Efficacy vs. VKA Non-Inferior Non-Inferior Bleeding vs. VKA Similar Similar DVT & PE DVT # Patients Treatment Duration Initial Therapy2 TTR VTE Type 1Apixaban data in VTE are not available, AMPLIFY & AMPLIFY-EXT are ongoing. 2Initial therapy in study group, both studies “bridged” control group. Prevention of Venous Thromboembolism in Orthopedic Surgery Patients Summary of Orthopedic VTE Data1 Comparator 1Includes Apixaban Dabigatran Edoxaban (2.5 mg q12h) (150 or 220mg/day) (30 mg/day) Rivaroxaban (10 mg/day) Enoxaparin 40 mg daily Superior Non-Inferior --- Superior Enoxaparin 20 mg q12h --- --- Superior --- Enoxaparin 30 mg q12h Non-Inferior Inferior --- Superior Bleeding vs. Enoxaparin Similar Similar Similar Similar patients undergoing both TKA and THA. Most studies excluded patients with CrCl < 30 ml/min. Summary of these data available in: Pharmacother 2011;31:1175-91. VTE Prophylaxis in Medical Patients Extended Apixaban vs. Standard Enoxaparin in Medical Patients • ADOPT Trial • Medical patients at high risk for VTE (n=4,495) • Treatment Groups – Apixaban 2.5 mg BID for 30 days – Enoxaparin 40 mg SQ daily during admission • Results – No difference in primary outcome • VTE death, PE, symptomatic DVT or asymptomatic proximal DVT (0.87; 0.62-1.23) – No difference in other outcomes • Did not meet criteria for non-inferiority (Key 2˚ outcome) – Higher rate of major bleeding (2.58; 1.02-7.24) • Summary – Extended apixaban was not better than standard enoxaparin in this superiority trial – Study was underpowered by over 2,250 patients (goal of 6,758) New Engl J Med 2011;365:2167-77. Extended Rivaroxaban vs. Standard Enoxaparin • Data not yet published • 8,101 medical patients • Treatment Groups – Rivaroxaban 10 mg daily for 35 days – Enoxaparin 40 mg SQ daily for 6-14 days • Primary Outcome Reduced – 0.77 (0.62-0.96) • Increased Major & Clinically Relevant Bleeding • No net clinical benefit http://www.theheart.org/article/1207331.do Acute Coronary Syndrome Apixaban Acute Coronary Syndrome (APPRAISE-2) • Double blind, placebo controlled, RCT • ACS plus 2 additional risk factors • Apixaban – 5 mg twice daily – 2.5 mg twice daily (CrCl < 40 ml/min) • No benefit in any efficacy outcomes • Safety – Major & minor TIMI, ISTH, and GUSTO criteria bleeding was increased with apixaban – Increased fatal and intracranial bleeding • Study terminated early at 1 year New Engl J Med 2011;365:699-708. Rivaroxaban • ATLAS-ACS 2 TIMI 51 Trial • All patients received low dose ASA with either: – – – – Rivaroxaban 2.5 mg twice daily or Rivaroxaban 5 mg twice daily Groups were stratified based on clopidogrel use Large number of patients from Eastern Europe (40%); however North America data were independently consistent/significant • Results – Primary Endpoint (CV Death, MI or CVA) • Both doses better than placebo – TIMI Major Bleeding • Rivaroxaban 2.5 mg BID vs Placebo: HR = 3.46 (2.08-5.77) • Rivaroxaban 5 mg BID vs Placebo: HR = 4.47 (2.71-7.36) – Intracranial Hemorrhage • Rivaroxaban 2.5 mg BID vs Placebo: HR = 2.83 (1.02-7.86) • Rivaroxaban 5 mg BID vs Placebo: HR = 3.74 (1.39-10.07) • Summary – Improved outcomes but 3-fold increased risk of major bleeding New Engl J Med 2012;366:9-19. Dabigatran • Phase II study completed & published 2/2011 • Meta-analysis (January 2012) – – – – 30,514 patients included Multiple indications/populations Majority of patients & events from RE-LY MI vs. Warfarin • RR: 1.33 (1.03-1.71) • AR: 0.40% – Mortality vs. Warfarin • RR: 0.89 (0.80-0.99) • AR: 0.19 – Interpret in light of stroke reduction – More investigation warranted Summary Take Home Points for Providers • Several new options currently or will exist to replace warfarin • Current approved indications include: – Prophylaxis of VTE in orthopedic patients – Prevention of stroke in patients with Afib • Date also exists to support their use in: – Treatment of VTE • Agents vary based on various pharmacologic and pharmacokinetic parameters – – – – None of the new agents require monitoring Would base choice of agent o patient specific factors All of them can be “measured” if needed Best technique for reversal is unknown for most at this time • Cost will be a limitation if not covered by insurance Practical Provider Information Parameter Dabigatran Rivaroxaban 150 mg BID (CrCl > 30 ml/min) 75 mg BID (CrCl 15-30 ml/min) 20 mg QD (CrCl > 50 ml/min) 15 mg QD (CrCl 15-50 ml/min) N/A 10 mg QD Primary Elimination Renal CYP3A4/P-gp1 Drug-Drug Interact. Rifampin, Dronedarone2, Ketoconazole2 CYP3A4/P-gp Inhibitors Timing of Dose Anytime (With or Without Food) 15, 20 mg Dose – With Food 10 mg Dose – Anytime aPTT PT Dyspepsia3 Well Tolerated3 Dosing Non-valvular Atrial Fibrillation Orthopedic VTE Px Practical Measuring Test Common Complaints 133% of elimination is renal and rivaroxaban does require dosage adjustment with CrCl < 50 ml/min. 2With dronedarone & ketoconazole when CrCl is 30-50 ml/min, decrease dabigatran to 75 mg BID; do not use when CrCl < 30 ml/min. 3As with any anticoagulant, adverse bleeding is still a concern. Both dabigatran and rivaroxaban demonstrated increased rates of GI bleeding, but lower rates of intracranial hemorrhage and similar overall rates of bleeding compared to warfarin. Practical Patient Information • Dabigatran – – – – – Store in original container Discard opened product after 4 months Dyspepsia most common side effect (up to 30%) Do not open capsule Comprehensive Patient Guide Available Online1 • Rivaroxaban – – – – 1Circ Ask pharmacist or physician about DDIs At Afib dose should take with supper Do not crush, chew, or split tablet Overall tolerated well 2011;124:e209-e211. (DOI: 10.1161/CIRCULATIONAHA.111.019786) Which of the following best describes your opinion regarding prescribing of dabigatran (Pradaxa®) or rivaroxaban (Xarelto®) after this program? 0% 0% e.. . pr no t Iw ill Ia m sti l lv er ... ib. .. 0% Im ay pr es cr be gin r.. . 0% Iw ill 1. I will begin routinely prescribing these agents. 2. I may prescribe them in a limited and select group of patients. 3. I am still very hesitant to prescribe them. 4. I will not prescribe them at all for now. New Developments in Oral Anticoagulants: Treating and Preventing Embolic Events in the 21st Century David Stewart, PharmD, BCPS Assistant Professor of Pharmacy Practice East Tennessee State University Bill Gatton College of Pharmacy stewardw@etsu.edu Use of Concomitant Antiplatelet Agents Antiplatelet Agents ARISTOTLE (Apixaban) RELY (Dabigatran) ROCKET-AF (Rivaroxaban) < 165 mg/day Yes < 100 mg/day Clopidogrel Yes Yes Yes Combination No Yes No Aspirin Use (%) 31% 40% 36% ASA New Engl J Med 2009;361:1139-51. New Engl J Med 2011;365:883-91. New Engl J Med 2011;365:981-92. Summary Table Parameter Apixaban Dabigatran Rivaroxaban Target Protein Factor Xa Thrombin (IIa) Factor Xa No Yes (etexilate) No CYP3A4/P-gp Renal CYP3A4/P-gp Avoid < 15 ml/min ↓ 15-29ml/min Avoid < 15 ml/min Avoid < 30 ml/min CYP3A4/P-gp Rifampin (P-gp) CYP3A4/P-gp Onset of activity 3-4 hrs 1-2 hrs 2-4 hrs t½ 8-15 hrs 12-18 hrs 5-9 hrs Twice daily Twice daily Daily Monitoring tests Anti-factor Xa ECT, TT, +/- aPTT Anti-factor Xa FDA Indications None Non-valvular Afib. Non-valvular Afib. Ortho VTE Proph. Afib Ortho VTE Proph Afib, VTE Afib, Ortho VTE Proph, VTE Pro-Drug 1˚ Elimination Renal Adjustment Drug-Drug Interact. Dosing interval Clinical Uses