Smells Unit Study Guide
advertisement
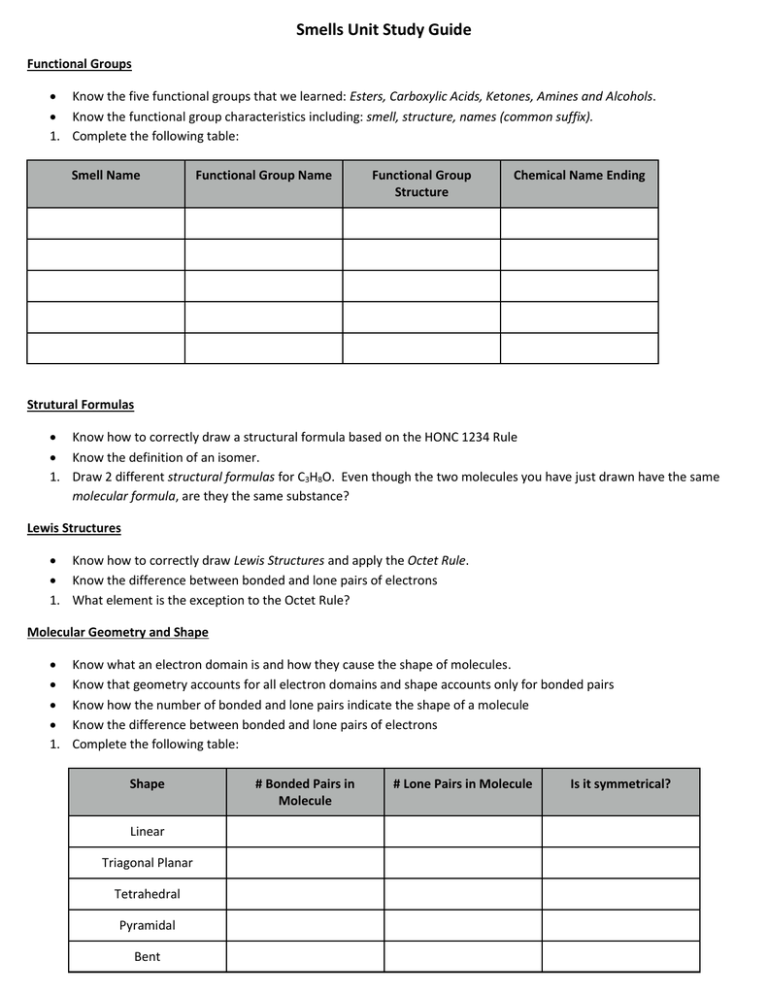
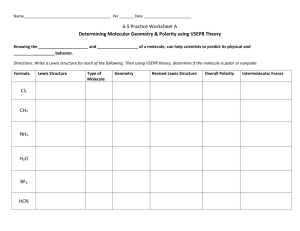
Smells Unit Study Guide Functional Groups Know the five functional groups that we learned: Esters, Carboxylic Acids, Ketones, Amines and Alcohols. Know the functional group characteristics including: smell, structure, names (common suffix). 1. Complete the following table: Smell Name Functional Group Name Functional Group Structure Chemical Name Ending Strutural Formulas Know how to correctly draw a structural formula based on the HONC 1234 Rule Know the definition of an isomer. 1. Draw 2 different structural formulas for C3H8O. Even though the two molecules you have just drawn have the same molecular formula, are they the same substance? Lewis Structures Know how to correctly draw Lewis Structures and apply the Octet Rule. Know the difference between bonded and lone pairs of electrons 1. What element is the exception to the Octet Rule? Molecular Geometry and Shape 1. Know what an electron domain is and how they cause the shape of molecules. Know that geometry accounts for all electron domains and shape accounts only for bonded pairs Know how the number of bonded and lone pairs indicate the shape of a molecule Know the difference between bonded and lone pairs of electrons Complete the following table: Shape Linear Triagonal Planar Tetrahedral Pyramidal Bent # Bonded Pairs in Molecule # Lone Pairs in Molecule Is it symmetrical? Electronegativity and Polarity Know what polarity is and the characteristics of polar/nonpolar molecules (smell, behavior as a drop, wand attraction, like dissolves like). Know how shape influences polarity. Know the pattern of electronegativity on the periodic table Be able to calculate the difference in electronegativity in a bond and use it to determine the type of bond. 1. What is the difference in electronegativity for the following: (a) NaCl (b) O2. What type of bond for each? Intermolecular Forces Know the differences between London Dispersion Forces, Dipole-Dipole and Hydrogen Bonds. SeCl4 CH2F2 CSe2 H2O Lewis Dot Structure Ball-n-Stick (show dipole arrows) Shape of Molecule # of Domains on Central Atom # of Lone Pairs on Central Atom Symmetrical shape? All surrounding atoms identical? Polar or Nonpolar? Dissolves in water? Will it smell? Type of IMF’s? **Final Note: This is a guide and is meant to help you focus you studying. Be sure to go over your making sense notes and homework. AS ALWAYS, COME IN AND GET HELP IF YOU NEED IT!