– Unit 5 Review Honors Chemistry
advertisement

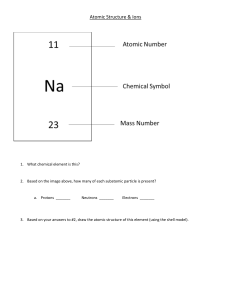
Name Date Pd Honors Chemistry – Unit 5 Review 1. For each of the following scientists, explain their contribution to the atomic theory. Be able to sketch the model of the atom they proposed, describe any experiments they conducted to lead to the development of their model, explain problems with the previous model their model addressed, and identify problems with their model that the next model addressed. a. Dalton b. J.J. Thomson c. E. Rutherford d. N. Bohr e. E. Schrodinger 2. Complete the following table from page 111 in your textbook: Particle Properties of Subatomic Particles Symbol Relative Charge Relative Mass Actual Mass a. Which subatomic particles are in the nucleus? b. Which subatomic particles have the same mass? c. Which subatomic particle is likely to be the "key player" in chemical reactions? Hint: Think about bonding! Modeling Chemistry 1 U5 hon review 2015 3. Complete the following table. Where “Symbol” is written, include the nuclide notation and any charge if the particle is an ion: Symbol 54Mn Atomic Number 35 Protons 2 19 Neutrons 2 20 18 Electrons Mass Number 79 4. How many protons are in the nucleus of a copper atom? How do you know? 5. What must have happened to a neutral strontium (Sr) atom to cause it to form strontium 2+ ion (Sr2+)? Explain your reasoning. 6. What must have happened to a neutral chlorine (Cl) atom to cause it to form a chlorine 1- ion (Cl1-)? Explain your reasoning. 7. The relative abundances of the isotopes of Element X in nature are: mass number = 204; 1.37% mass number = 206; 26.26% mass number = 207; 20.82% mass number = 208; 51.55% Calculate the average atomic mass and identify the element. Write the nuclide notation of each isotope. Modeling Chemistry 2 U5 hon review 2015