– Unit 5 Review General Chemistry
advertisement

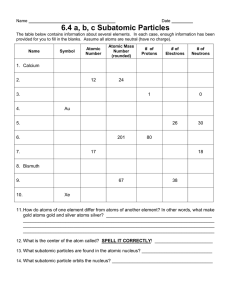
Name Date Pd General Chemistry – Unit 5 Review 1. For the following scientists, describe the atomic model they proposed and their contributions to the progression of the atomic theory. a. Dalton b. J.J. Thomson c. E. Rutherford 2. Complete the following table from page 111 in your textbook: Particle Properties of Subatomic Particles Symbol Relative Charge Relative Mass Actual Mass a. Which subatomic particles are in the nucleus? b. Which subatomic particles have the same mass? c. Which subatomic particle is the "key player" in chemical reactions? Hint: Think about bonding! Modeling Chemistry 1 U5 gen Review 2010 3. Complete the following table: Symbol 54Mn Atomic Number 35 Protons 2 19 Neutrons 2 20 18 Electrons Mass Number 79 4. How many protons are in the nucleus of a copper atom? 5. Has a strontium 2+ ion (Sr2+) lost or gained electrons? 6. What is the symbol and charge of chlorine when it becomes an ion? 7. The relative abundances of the isotopes of Element X in nature are: mass number = 204; 1.37% mass number = 206; 26.26% mass number = 207; 20.82% mass number = 208; 51.55% Calculate the average molar mass and identify the element. 8. What are the types of sublevels and number of orbitals in the following energy levels? a. n = 1 b. n = 2 c. n = 3 d. n = 4 Modeling Chemistry 2 U5 gen Review 2010 9. Sketch an orbital spin diagram for the following atoms: a. C b. K c. S d. Ar What useful information is conveyed in an orbital spin diagram? 10. Write the electron configuration of each element, use shorthand notation for elements with atomic number greater than 36: a. hydrogen b. barium c. krypton d. vanadium e. bromine f. arsenic g. magnesium h. sulfur i. radon Modeling Chemistry 3 U5 gen Review 2010