Name____________________________ Date______ Period__
advertisement
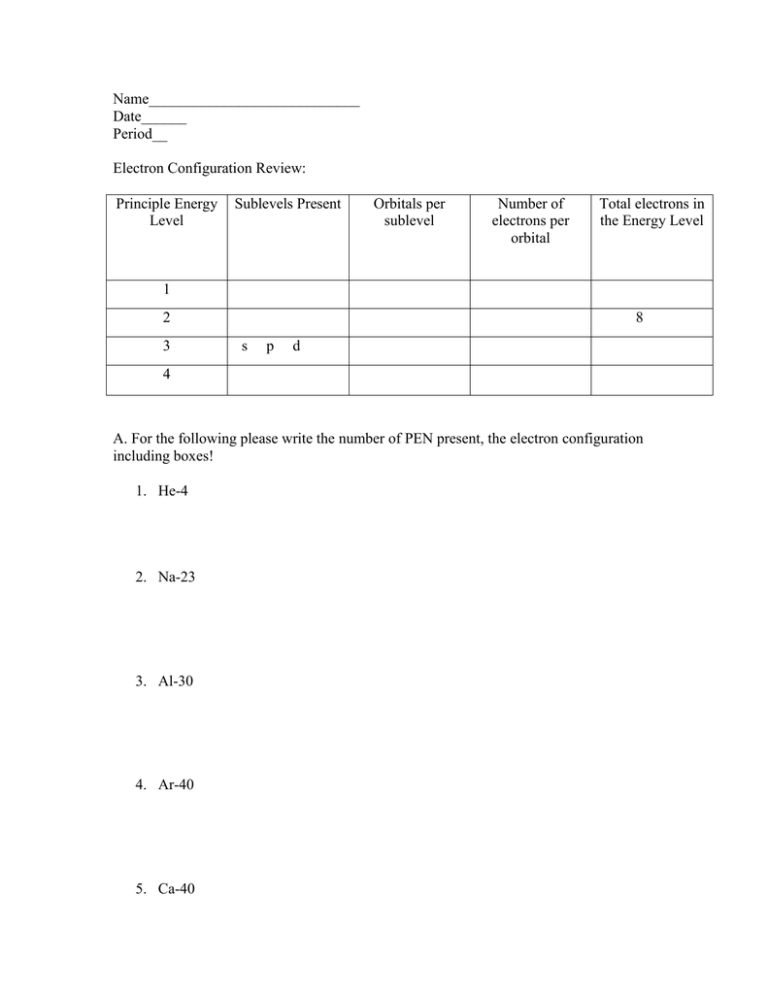
Name____________________________ Date______ Period__ Electron Configuration Review: Principle Energy Level Sublevels Present Orbitals per sublevel Number of electrons per orbital Total electrons in the Energy Level 1 2 3 8 s p d 4 A. For the following please write the number of PEN present, the electron configuration including boxes! 1. He-4 2. Na-23 3. Al-30 4. Ar-40 5. Ca-40 B. For the following fill in the electrons into the boxes according to the information provided. Answer any accompanying questions. Remember each box represents an orbital for one of the sublevels in the electron cloud. P3 Li-7 E 3 1s22s1 N4 1s 2s 1. How many sublevels contain electrons? 2. Of these sublevels, how many are filled to their maximum capacity? 3. Which sublevels are filled completely? 4. Which sublevels are NOT filled completely? 5. What is the outermost (valence shell) energy level in an atom of Li? 6. How many valence electrons are present? Circle the sublevel(s) that contain the valence electrons. P7 N-14 E 7 N7 1s22s22p3 1s 2s 2p 7. How many sublevels contain electrons? 8. Of these, how many are completely filled? Name them. 9. Which sublevels are HALF filled? 10. How many orbitals are half-filled? 11. What is the valence shell of the electron cloud in N-14? 12. How many valence electrons are present? Circle the sublevel(s) that contain them. Finish the electron configuration and boxes. Remember Aufbau’s Principle: P 19 K-39 E 19 1s22s22p63s23p6___ N 20 1s 2s 2p 3s 3p 13. How many completely filled sublevels are present? 14. How many completely filled orbitals are present? 15. How many orbitals are half filled? 16. How many energy levels contain electrons? 17. How many valence electrons are in K-39? Circle the sublevels that contain the valence electron(s). P V-51 E N 18. How many completely filled sublevels are present? 19. How many completely filled orbitals are present? 20. How many orbitals are half filled? 21. How many energy levels contain electrons? 22. How many valence electrons are in K-39? Circle the sublevels that contain the valence electron(s). Define the Octet Rule: Define valence electron: Bonding: Chemical Bonding is an event that occurs between two atoms. In a chemical bond the electrons from each atom are attracted to the nucleus the opposite atom, and as a result a bond is formed. Think of a chemical bond as a force of attraction between two atoms, that holds them together. Chemical bonds form because of electrons. Specifically, it is the valence electrons that are involved in chemical bonds. Remember the octet rule: An atom is stable (happy) when it has 8 valence electrons. An unstable atom (anything that has less than 8 valence) is considered reactive. In other words, it will do what it takes to reach 8 valence! One of the best ways to visualize why bonding occurs is to use the Lewis Dot structures. If you recall Lewis Dot structures are represented with the symbol of the element and surrounding the 4 sides of the symbol dots are placed to represent the number of valence electrons present. Please draw the Lewis Dot Structures for: H Li Be B C N O F Ne Na Mg Al Si P S Cl Ar There are two major types of bonds that can form between atoms: 1. Ionic Bonds. In an ionic bond the valence electron(s) from one atom is/are transferred to the second atom. Ionic bonds involve the formation of IONS. To break it down even further, one atom gains and electron(s) and one atom loses an electron(s). Why? Remember, the atoms want to reach the magic number of 8 valence! By giving up electrons or gaining them they reach their goal. How? Follow the steps below. Step 1. A sodium atom begins with 1 valence – no where near 8. However, count how many electrons are in the second energy level… Step 2. Chlorine starts with 7 valence. That’s almost 8. If only it can get one more it would have 8 valence in the 3rd energy level. Step 3. If you guessed correctly, the sodium atom is eager to give up its one valence electron to chlorine. If it loses an electron, sodium is now a positively charged ion. Why? Sodium would then have a valence of 8 (in the second energy level). Also chlorine would then complete its 3rd principle energy level with 8 by gaining this electron, making it a negatively charged ion. Both attain the octet rule with a little give and take. Step 4. The transfer of electrons from one atom to another forms a chemical bond between the two, “locking” them together as 1 compound. (Remember what a compound is?) 2. Covalent Bonds. In a covalent bond, electrons are not transferred from one atom to another. Instead, electrons are shared between two atoms. Think of it as though the two atoms are holding electron hands. A. Each atom contributes an electron to an “electron pair”. This pair is shared by both atoms and allows them to feel as though they have an extra electron. In this way both atoms can reach a valence of 8. Step 1. In each atom of chlorine there are 7 valence. Both so close to 8! Would either be willing to give up a valence? The answer is no. Step 2. So they compromise. If each share one electron with the other, then each would feel as though 8 valence were present. B. In some cases more than one pair of electrons can be shared between the same two atoms. Multiple pairs can be formed between the same to atoms if it helps them attain 8 valence. Using the hand-holding metaphor, the two atoms are holding hands in a couple of places. Each Oxygen atom has 6 valence, close to the octet rule. Neither is willing to lose electrons. If one pair is shared then both feel as those there are 7 valence. If a second pair of electrons is shared, then both feel as though the have 8 valence. Both are now happy. Not yet 8. C. In some cases one atom may form covalent bonds with more than one other element. In other words, some atoms will hold hands with multiple partners at the same time.