Ionic Solids Characteristics
advertisement

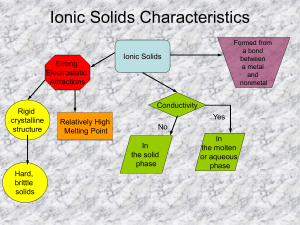
Ionic Solids Characteristics Formed from a bond between a metal and nonmetal Ionic Solids Strong Electrostatic Attractions Rigid crystalline structure Conductivity Relatively High Melting Point Yes No In the solid phase Hard, brittle solids In the molten or aqueous phase The First Couple • # 1) Energy is released when bonds are formed. Energy is absorbed when bonds are broken. • #2) As a bond forms the PE decreases. • #3) Energy is released -> stability increases • #4) Two atoms combine to form a molecule, bond formed, energy is released #5 Which electron-dot diagram represents H2? Both hydrogen’s will equally share their valence electron H H H H #9) In which compound do the atoms have the greatest difference in electronegativity? (1) NaBr |0.9 – 3.0| = 2.1 (2) AlCl3 |1.6 – 3.2| = 1.6 (3) KF |0.8 – 4.0| = 3.2 (4) LiI |1.0 – 2.7| = 1.7 #13) Given the reaction: M + 2H2O -> M(OH)2 + H2 The metal represented by M is most likely a metal from Group The subscript of 2 on the hydroxide came from the charge on the metal (M). M(OH)2 +2 -1 M (OH) So the Metal (M) must be from Group 2 because of its +2 oxidation state! #16) Element X has an electron configuration of 2-8-3. This element will combine with the phosphate ion to form a compound with the formula 3 Valence e-’s means that is will have an oxidation state of +3 +3 X (PO4) -3 Criss Cross w/out charges X3(PO4)3 3 Reduce to Lowest Ratio X (PO4) 3 From Table E: phosphate has a -3 #33 a. H H b. H H c. 1 Mg + H2 is nonpolar and H2O is polar, like dissolves like, therefore H2 will not dissolve in H2O. 1 H2SO4 1 H2 + 1 MgSO4 #34 a. HNH H b. H HNH H + #35 a. b. H Cl + H Cl c. H-Cl is polar, water is polar; like dissolves like #36 a. N N OR N N b. N2 is very stable, unreactive because of triple bond (a lot of energy was released when this bond was formed). #6) Group 1 metals are highly reactive and form stable compounds. #7) Na 1s22s22p63s1 Will become [Na]+1 Na Cl 1s22s22p63s23p5 Cl 1s22s22p6 Ne Will become 1s22s22p63s23p6 Ar Cl -1 #8. Radon is a group 2 (metal), it wants to lose its 2 valence electrons to a nonmetal. 1) Iodine – nonmetal, wants to gain 1 e2) Fluorine – nonmetal, wants to gain 1 e3) sodium – metal, wants to lose 1 e4) calcium – metal, wants to lose 2 eOf the 2 choices that want electrons choice 2 is more reactive, larger electronegativity, than choice 1. Fluorine is the answer (choice 2). #10) Which compound would most likely have the greatest ionic character? (1) CO |2.6 – 3.5| = 0.9 (2) KF |0.8 – 4.0| = 3.2 (3) CaO |1.0 – 3.5| = 2.5 (4) LiH |1.0 – 2.1| = 1.1 #11) Given the reaction H2 + Cl2 → 2HCl • Bond Breaking requires/absorbs energy. • Bond Formation releases/emits energy. The H2 and Cl2 bonds are broken – requires energy! The HCl bond is formed- releases energy! The answer is choice (4) the forming of the HCl bond releases energy #12) Noble gases have the most stable electron configuration, choice 1 (Ne). #14) In order for the electrons to have been transferred to Oxygen, the oxygen will have to be the most electronegative element and the ionic character of the compound would be 1.7 or greater. (1) CO2 (2) N2O |2.6 – 3.5| = 0.9 |3.0 – 3.5| = 0.5 (2) NO2 |3.0 – 3.5| = 0.5 (4) Na2O |0.9 – 3.5| = 2.6 15) Compounds including polyatomic ions have covalent bonding within and ionic bonding to the metal outside the parenthesis (brackets). 2- Ca 2+ O O SO O