VOLTAIC CELL: A SPONTANEOUS ENERGY RELEASING REDOX
advertisement

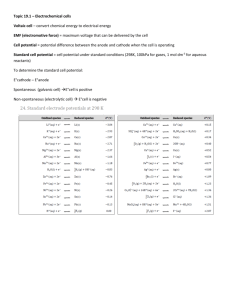
VOLTAIC CELL: A SPONTANEOUS ENERGY RELEASING REDOX REACTION…USING Fe AND Pb ELECTRONS RELEASED BY REDUCING AGENT(THAT WHICH IS OXIDIZED-Fe(s) NEGATIVE SALT BRIDGE ION CARRIES NEGATIVE CHARGE BACT TO ANODE, THIS IS THE IONIC HALF OF THE CIRCUIT. ELECTRONS GAINED BY OXIDIZING AGENT IONS IN CATHODAL SOLUTION (Pb+2 (aq) ) THE CATHODE ITSELF IS Pb SOLID. ELECTRONS REPELLED FROM ANODE INTO WIRE, ELECTRONS CARRY NEGATIVE CHARGE THROUGH WIRE , RELEASE CHEMICAL POTENTIAL ENERGY AS ELECTRICITY VOLTAIC CELL VOLTMETER SLIDE ONE OVERVIEW ANIMATION SO4 SO4 2- 2- Na+ Na+ SO42Na+ Na+ 2e- - Fe Fe 2eFe2+ SO42- Fe0(s) 2e- + Fe2+ ANODE (ANOX) 2+ PbPb Pb Na+ Na+ Pb2+ Pb + + + Pb 2e- + Pb2+ Pb0(s) CATHODE (REDCAT) ANODE LIST Fe0(s) 2e- + Fe2+ F0 IS: 1) 2) 3) 4) 5) 6) OXIDIZED IS THE ANODE TOPMOST ON TABLE J. IS ELECTRON SOURCE IS REDUCING AGENT IS COVERTED TO AN AQUIOUS ION. THE ANODE: 1) IS NEGATIVE DUE TO ELECTRONS BEING PRODUCED. 1) DISSOLVES…LOSES MASS 2) THE ANODAL SOLUTION BECOMES + DUE TO PRODUCTION OF A POSITIVE ION…SALT BRIDGE – ION NEUTRALIZES. CATHODE LIST 2e- + Pb2+ Pb0(s) Pb+2 IS: 1) 2) 3) 4) 5) 6) REDUCED REACTS AT CATHODE (Pb 0) BOTTOM MOST ON TABLE J. IS ELECTRON DESTINATION IS OXIDIZING AGENT AGENT ITS AQUIOUS ION IS SOLIDIFIED. THE CATHODE: 1) IS POSITIVE DUE TO ELECTRONS BEING CONSUMED. 1) ELECTROPLATED…GAINS MASS 2) THE CATHODAL SOLUTION BECOMES - DUE TO CONSUMPTION OF A POSITIVE ION…SALT BRIDGE + ION NEUTRALIZES. ELECTRON FLOW - ELECTRODES: FROM ANODE (SOLID IRON Fe) TRANSFERS ELECTRONS TO CATHODE (SOLID LEAD Pb). -SPECIES: FROM Fe0 TO Pb+2 THE CIRCUIT -THE WIRE:THE FLOW OF ELECTRONS FROM ANODE TO CATHODE IS ELECTRICAL CONDUCTION, - CHARGE LEAVES ANODE CARRIED BY ELECTRONS. -SALT BRIDGE: NEGATIVE CHARGE RETURNS TO ANODE AS NEGATIVE IONS. NOTE ♪, ELECTRONS NEVER TRAVEL IN SALT BRIDGE OR ANY SOLUTION. VOLTAIC AS THE Fe(S) IS CELL OXIDIZED, IT DISSOLVES AND SLIDE TWO THE ANODE LOSES MASS AND THE ANODE 2e- ARE AS THE Fe(S) IS OXIDIZED, IT DISSOLVES AND THE VOLTMETER ANODE LOSES MASS, THE IRON(II) IONS WILL MAKE THE ANODE SOLUTION POSITIVE, THEREFORE THE + Na NEGATIVE SALT SO42- Na+ 2SO 4 SO42Na+ Na+BRIDGE ION MOVES IN TO NEGATE THE + CHARGE RELEASED 2eFe Fe Fe2+ SO4 Fe0(s) 2e- + Fe2+ ANODE (ANOX) 2- NSBTA Negative salt bridge to anode Pb2+ Never sell booze to adolescents! Pb Pb 2e- + Pb2+ Pb0(s) CATHODE (REDCAT) VOLTAIC CELL VOLTMETER SLIDE THREE e- FLOW 2eFe Fe Fe0(s) 2e- + ELECTRONS ALWAYS MOVE FROM Na ANODE + 2SOCATHODE TO 4 Na+ SO42(FATCAT). THE Na+ Na+ ELECTRONS BUILD UP IN THE ANODE Fe2+ MAKING IT NEGATIVE, TRAVEL TO THE SO42-CATHODE WHERE A METAL ION GAINS Pb2+ THEM IN REDUCTION, THE CATHODE IS POSITIVE. VOLTMETER ALWAYS Fe2+ POINTS TO CATHODE, 2e + ANODE (ANOX) 2ePb Pb Pb2+ Pb0(s) CATHODE (REDCAT) VOLTAIC CELL VOLTMETER SLIDE FOUR THE CATHODE THE POSITIVE SALT ION MIGRATES TO THE CATHODE TO REPLACE + CHARGE LOST AS Pb(II) Na+ 2SO4 Na+ ARE REDUCED TO2-Pb SO42SOLID.SO4 Na+ Na+ 2eFe Fe 2eFe2+ SO42AS ELECTRONS ARE GAINED BY Pb(II) IONS FROM THE CATHODE, THE IONS BECOME REDUCED INTO Pb(S) Fe0(s) 2e- + Fe2+ ANODE (ANOX) Pb2+ Pb Na+ Na+ Pb Pb2+ Pb THE SOLID LEAD BONDS (METALLIC BONDING) THE CATHODE WHICH GAINS MASS. THIS IS ELECTROPL ATING. 2e- + Pb2+ Pb0(s) CATHODE (REDCAT)