Calorimetry more Q's ws
advertisement

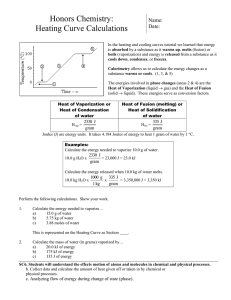
Name _________________________ Period _________________ Calorimetry (more Q’s) Worksheet Model 1: | | Q5 = _________ | | _____________________________ Q4= _________ | | Temperature | oC | Q3= _________ | | Q2 = _________ | | _________________ | | / Q1 = _________ |/ |________________________________________________ Energy (Joules) added or Time 1. In model 1, this region (1) of solid water uses the formula Q = m Cp solid T in order to determine the amount of heat energy required to change the temperature of the solid ice over that region. Write this formula on the line next to Q1 The Cp of solid water = 2.03 J/ g C 2. Does the temperature change for this region of the heating curve? ______________ 3. Calculate the Q of 50.0 g of ice from – 10 C to 0 C. Show all calculations. 4. In model 1, the region (2) with the liquid-solid water equilibrium uses the formula Q = m Hfusion to calculate the amount of heat energy required to change the solid water to liquid water. Place this formula on the line next to Q2. H fusion= 333.55 J/g (heat of fusion of ice) 5. Does the temperature change in this region (2) of the heating curve? _____________What is the temperature change for liquid water in this region? _____________________ 6. Calculate the Q2 of 50.0 g of ice all changing to liquid water. Show all calculations. 7. In model 1, this region (3) of liquid water uses the formula Q3 = m Cp liquid T isused in order to determine the amount of heat energy required to change the temperature of the liquid water over this region. Write this formula on the line next to Q3 The Cp of liquid water = 4.18 J/ g C 8. Does the temperature change for this region of the heating curve? ______________ What is the temperature change for liquid water in this region? _____________________ 9. Calculate the Q3 of liquid water for this region (3). Show all calculations. 10. In model 1, the region (4) with the liquid-vapor water equilibrium uses the formula Q4 = m H vaporization to calculate the amount of heat energy required to change the liquid water to gaseous water. Place this formula on the line next to Q4. H vaporization = 2257 J/g (heat of vaporization of water) 11. Does the temperature change in this region (4) of the heating curve? _____________What is the temperature change for liquid water in this region? _____________________ 12. Calculate the Q4 of 50.0 g of liquid water. Show all calculations. 13. In model 1, this region (5) of gaseous water uses the formula Q5 = m Cp vapor T in order to determine the amount of heat energy required to change the temperature of the gaseous water over that region. Write this formula on the line next to Q5. The Cp of gaseous water = 2.02 J/gC 14. Does the temperature change for this region of the heating curve? ______________ What is the temperature change for gaseous water in this region? _____________________ 15. Calculate the Q5 of 50.0 g of gaseous water from 100.0 C to 110.0 C. Show all calculations.